Formulation and evaluation of injectable dextran sulfate sodium nanoparticles as a potent antibacterial agent
- PMID: 33972626
- PMCID: PMC8110975
- DOI: 10.1038/s41598-021-89330-0
Formulation and evaluation of injectable dextran sulfate sodium nanoparticles as a potent antibacterial agent
Abstract
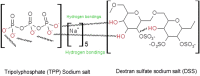
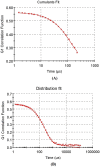
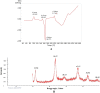
The purpose of this study was to develop a novel nano antibacterial formulation of dextran sulfate sodium polymer. The dextran sulfate sodium (DSS) nanoparticles were formulated with gelation technique. The nanoparticles exhibited significant physicochemical and effective antibacterial properties, with zeta potential of - 35.2 mV, particle size of 69.3 z d nm, polydispersity index of 0.6, and percentage polydispersity of 77.8. The DSS nanoparticles were stable up to 102 °C. Differential scanning calorimetry revealed an endothermic peak at 165.77 °C in 12.46 min, while XRD analysis at 2θ depicted various peaks at 21.56°, 33.37°, 38.73°, 47.17°, 52.96°, and 58.42°, indicating discrete nanoparticle formation. Antibacterial studies showed that the DSS nanoparticles were effective against Gram-positive and Gram-negative bacteria. The minimum inhibitory concentrations of DSS nanoparticles for Bacillus subtilis (B. subtilis), Staphylococcus aureus (S. aureus), Streptococcus pyogenes (S. pyogenes), Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), Klebsiella pneumoniae (K. pneumoniae) and Proteus vulgaris (P. vulgaris) were 150, 200, 250, 150, 200, 250, 250 µg/mL, respectively. The antibacterial effects of DSS nanoparticles were in the order E. coli (26 ± 1.2 mm) at 150 µg/mL > S. pyogenes (24.6 ± 0.8 mm) at 250 µg/mL > B. subtilis (23.5 ± 2 mm) at 150 µg/mL > K. pneumoniae (22 ± 2 mm) at 250 µg/mL > P. aeruginosa (21.8 ± 1 mm) at 200 µg/mL > S. aureus (20.8 ± 1 mm) at 200 µg/mL > P. vulgaris (20.5 ± 0.9 mm) at 250 µg/mL. These results demonstrate the antibacterial potency of DSS injectable nanoparticles.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Safhi MM, et al. Therapeutic potential of chitosan nanoparticles as antibiotic delivery system: challenges to treat multiple drug resistance. Asian J. Pharm. 2016;10(2):S61–S66. doi: 10.22377/ajp.v10i2.624. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

