Modifications of liver stiffness and CXCL4, TGF-β1 and HGF are similar in HCV- and HIV/HCV-infected patients after DAAs
- PMID: 33972651
- PMCID: PMC8110591
- DOI: 10.1038/s41598-021-89370-6
Modifications of liver stiffness and CXCL4, TGF-β1 and HGF are similar in HCV- and HIV/HCV-infected patients after DAAs
Abstract
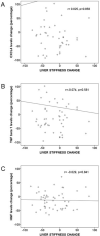
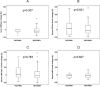
The objective of this work was to identify predictive factors of fibrosis regression after direct antiviral agents (DAAs) in HCV-monoinfected and HIV/HCV-coinfected patients. This was a prospective study of HCV-monoinfected (n = 20), HIV/HCV-co-infected (n = 66) patients and healthy controls (n = 15). Patients had started DAAs and achieved sustained virological response. Liver stiffness (LS) and serum concentrations of profibrotic transforming growth factor (TGF)-β1 and CXC chemokine ligand 4 (CXCL4) and antifibrotic HGF hepatocyte growth factor (HGF) were analyzed at baseline (M0) and 12 months after starting DAAs (M12). A M12 LS achievement of ≤ 9.5 kPa was considered the cutoff point to discharge from a liver clinic. The LS decrease from M0 to M12 was 34%. No significant differences were observed in LS decline between HCV- and HIV/HCV-infected individuals. Changes of serum CXCL4, TGF-β1 and HGF levels did not correlate with LS improvement. 16 out from 56 patients (28%) with a baseline LS > 9.5 achieved a M12 LS ≤ 9.5. HCV-monoinfected and HIV/HCV coinfected patients experienced a significant reduction of LS after sustained virological response. This improvement did not correlate with changes in serum profibrotic or antifibrotic markers. A 29% of those with a baseline LS > 9.5 achieved a LS under this cutoff point.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Changes in liver stiffness and non-invasive markers in chronic hepatitis C virus patients with and without HIV co-infection following interferon-free antiviral treatment.Arab J Gastroenterol. 2025 May;26(2):212-218. doi: 10.1016/j.ajg.2025.02.006. Epub 2025 May 6. Arab J Gastroenterol. 2025. PMID: 40335375
-
Antiretroviral therapy and sustained virological response to HCV therapy are associated with slower liver fibrosis progression in HIV-HCV-coinfected patients: study from the ANRS CO 13 HEPAVIH cohort.Antivir Ther. 2012;17(7):1335-43. doi: 10.3851/IMP2419. Epub 2012 Oct 10. Antivir Ther. 2012. PMID: 23052829
-
Liver Stiffness-Based Strategies Predict Absence of Variceal Bleeding in Cirrhotic Hepatitis C Virus-Infected Patients With and Without Human Immunodeficiency Virus Coinfection After Sustained Virological Response.Clin Infect Dis. 2021 Mar 1;72(5):e96-e102. doi: 10.1093/cid/ciaa1726. Clin Infect Dis. 2021. PMID: 33211801
-
Are there any challenges left in hepatitis C virus therapy of HIV-infected patients?Int J Antimicrob Agents. 2020 Jul;56(1):105527. doi: 10.1016/j.ijantimicag.2018.08.019. Epub 2018 Aug 23. Int J Antimicrob Agents. 2020. PMID: 30145247 Review.
-
Treatment of chronic HCV genotype 1 coinfection.Curr HIV/AIDS Rep. 2015 Sep;12(3):326-35. doi: 10.1007/s11904-015-0278-4. Curr HIV/AIDS Rep. 2015. PMID: 26228050 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

