Structural analogues in herbal medicine ginseng hit a shared target to achieve cumulative bioactivity
- PMID: 33972672
- PMCID: PMC8110997
- DOI: 10.1038/s42003-021-02084-3
Structural analogues in herbal medicine ginseng hit a shared target to achieve cumulative bioactivity
Abstract
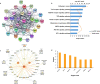
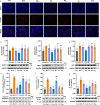
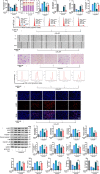
By a pilot trial on investigating immunomodulatory activity and target of ginsenosides, the major bioactive components of ginseng, here we report that structural analogues in herbal medicines hit a shared target to achieve cumulative bioactivity. A ginsenoside analogues combination with definite immunomodulatory activity in vivo was designed by integrating pharmacodynamics, serum pharmacochemistry and pharmacokinetics approaches. The cumulative bioactivity of the ginsenoside analogues was validated on LPS/ATP-induced RAW264.7 macrophages. The potentially shared target NLRP3 involved in this immunomodulatory activity was predicted by systems pharmacology. The steady binding affinity between each ginsenoside and NLRP3 was defined by molecular docking and bio-layer interferometry assay. The activation of NLRP3 inflammasomes in LPS/ATP-induced RAW264.7 was significantly suppressed by the combination, but not by any individual, and the overexpression of NLRP3 counteracted the immunomodulatory activity of the combination. All these results demonstrate that the ginsenoside analogues jointly hit NLRP3 to achieve cumulative immunomodulatory activity.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Ginsenoside Rc from Korean Red Ginseng (Panax ginseng C.A. Meyer) Attenuates Inflammatory Symptoms of Gastritis, Hepatitis and Arthritis.Am J Chin Med. 2016;44(3):595-615. doi: 10.1142/S0192415X16500336. Epub 2016 Apr 24. Am J Chin Med. 2016. PMID: 27109153
-
Ginsenoside Rg3 suppresses the NLRP3 inflammasome activation through inhibition of its assembly.FASEB J. 2020 Jan;34(1):208-221. doi: 10.1096/fj.201901537R. Epub 2019 Nov 20. FASEB J. 2020. PMID: 31914640
-
Sulfur fumigation reducing systemic exposure of ginsenosides and weakening immunomodulatory activity of ginseng.J Ethnopharmacol. 2017 Jan 4;195:222-230. doi: 10.1016/j.jep.2016.11.023. Epub 2016 Nov 15. J Ethnopharmacol. 2017. PMID: 27856301
-
Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer.Acta Pharmacol Sin. 2008 Sep;29(9):1109-18. doi: 10.1111/j.1745-7254.2008.00869.x. Acta Pharmacol Sin. 2008. PMID: 18718180 Review.
-
Ginseng pharmacology: multiple constituents and multiple actions.Biochem Pharmacol. 1999 Dec 1;58(11):1685-93. doi: 10.1016/s0006-2952(99)00212-9. Biochem Pharmacol. 1999. PMID: 10571242 Review.
Cited by
-
Nardostachys jatamansi and levodopa combination alleviates Parkinson's disease symptoms in rats through activation of Nrf2 and inhibition of NLRP3 signaling pathways.Pharm Biol. 2023 Dec;61(1):1175-1185. doi: 10.1080/13880209.2023.2244176. Pharm Biol. 2023. PMID: 37559448 Free PMC article.
-
Exploring the pathways of drug repurposing and Panax ginseng treatment mechanisms in chronic heart failure: a disease module analysis perspective.Sci Rep. 2024 May 27;14(1):12109. doi: 10.1038/s41598-024-61926-2. Sci Rep. 2024. PMID: 38802411 Free PMC article.
-
Uncoupled pyroptosis and IL-1β secretion downstream of inflammasome signaling.Front Immunol. 2023 Apr 6;14:1128358. doi: 10.3389/fimmu.2023.1128358. eCollection 2023. Front Immunol. 2023. PMID: 37090724 Free PMC article. Review.
-
Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1.Open Life Sci. 2025 Aug 5;20(1):20251109. doi: 10.1515/biol-2025-1109. eCollection 2025. Open Life Sci. 2025. PMID: 40771411 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

