Integrating barcoded neuroanatomy with spatial transcriptional profiling enables identification of gene correlates of projections
- PMID: 33972801
- PMCID: PMC8178227
- DOI: 10.1038/s41593-021-00842-4
Integrating barcoded neuroanatomy with spatial transcriptional profiling enables identification of gene correlates of projections
Abstract
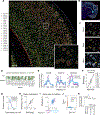
Functional circuits consist of neurons with diverse axonal projections and gene expression. Understanding the molecular signature of projections requires high-throughput interrogation of both gene expression and projections to multiple targets in the same cells at cellular resolution, which is difficult to achieve using current technology. Here, we introduce BARseq2, a technique that simultaneously maps projections and detects multiplexed gene expression by in situ sequencing. We determined the expression of cadherins and cell-type markers in 29,933 cells and the projections of 3,164 cells in both the mouse motor cortex and auditory cortex. Associating gene expression and projections in 1,349 neurons revealed shared cadherin signatures of homologous projections across the two cortical areas. These cadherins were enriched across multiple branches of the transcriptomic taxonomy. By correlating multigene expression and projections to many targets in single neurons with high throughput, BARseq2 provides a potential path to uncovering the molecular logic underlying neuronal circuits.
Conflict of interest statement
Competing Interests
A.M.Z. is a founder and equity owner of Cajal Neuroscience and a member of its scientific advisory board. The remaining authors declare no competing interests.
Figures

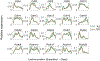








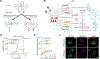





References
Method References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

