Factorial design-assisted reversed phase-high performance liquid chromatography method for simultaneous determination of fluconazole, itraconazole and terbinafine
- PMID: 33972882
- PMCID: PMC8103232
- DOI: 10.1098/rsos.202130
Factorial design-assisted reversed phase-high performance liquid chromatography method for simultaneous determination of fluconazole, itraconazole and terbinafine
Abstract
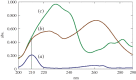
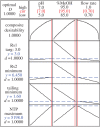
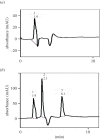
A 23 full factorial design model was used for the development of a new high performance liquid chromatography method with UV detection to estimate three antifungal drugs simultaneously. Fluconazole (FLU), itraconazole (ITR) and terbinafine (TRH) are co-administered for severe fungal infections. They have been determined using MOS-1 Hypersil C18 column and an isocratic eluent; methanol 95% and phosphate buffer 5% with 0.001% triethylamine. The pH was adjusted to 7, and the flow rate was 0.7 ml min-1. The three drugs were separated within less than 7 min at 210 nm. The developed method gave a linear response over 5-80 µg ml-1, 5-50 µg ml-1 and 1-50 µg ml-1 for FLU, ITR and TRH, respectively. It showed detection limits of 0.88, 0.29 and 0.20 µg ml-1 and quantification limits of 2.66, 0.88 and 0.60 µg ml-1 for the three drugs, respectively. The design of the experiment facilitated the optimization of different variables affecting the separation of the three drugs. The sensitivity of the designed method permitted the simultaneous estimation of ITR and TRH in spiked human plasma successfully.
Keywords: HPLC; factorial; fluconazole; isocratic; itraconazole; terbinafine.
© 2021 The Authors.
Figures




Similar articles
-
Simple HPLC method for simultaneous estimation of fluconazole and tinidazole in combined dose tablet.J Chromatogr Sci. 2009 Nov-Dec;47(10):885-8. doi: 10.1093/chromsci/47.10.885. J Chromatogr Sci. 2009. PMID: 19930800
-
Hydro-organic mobile phase and factorial design application to attain green HPLC method for simultaneous assay of paracetamol and dantrolene sodium in combined capsules.BMC Chem. 2023 Aug 2;17(1):92. doi: 10.1186/s13065-023-00990-7. BMC Chem. 2023. PMID: 37533125 Free PMC article.
-
Combining derivative and synchronous approaches for simultaneous spectrofluorimetric determination of terbinafine and itraconazole.R Soc Open Sci. 2020 Aug 5;7(8):200571. doi: 10.1098/rsos.200571. eCollection 2020 Aug. R Soc Open Sci. 2020. PMID: 32968519 Free PMC article.
-
Simultaneous Determination of Six Benzodiazepines in Spiked Soft Drinks by High Performance Liquid Chromatography with Ultra Violet Detection (HPLC-UV).Iran J Pharm Res. 2016 Spring;15(2):457-63. Iran J Pharm Res. 2016. PMID: 27642316 Free PMC article.
-
Pharmacokinetics of antifungal agents in onychomycoses.Clin Pharmacokinet. 2001;40(6):441-72. doi: 10.2165/00003088-200140060-00005. Clin Pharmacokinet. 2001. PMID: 11475469 Review.
Cited by
-
Deduction of full factorial design of HPLC technique for the simultaneous analysis of meloxicam and esomeprazole in their laboratory prepared tablets.Sci Rep. 2025 Apr 15;15(1):12922. doi: 10.1038/s41598-025-95706-3. Sci Rep. 2025. PMID: 40234474 Free PMC article.
-
Design-assisted HPLC-UV method for therapeutic drug monitoring of pholcodine, ephedrine, and guaifenesin in biological fluids.Sci Rep. 2024 Nov 14;14(1):27933. doi: 10.1038/s41598-024-78793-6. Sci Rep. 2024. PMID: 39537794 Free PMC article.
-
Implementation of HILIC-UV technique for the determination of moxifloxacin and fluconazole in raw materials and pharmaceutical eye gel.Sci Rep. 2022 Aug 4;12(1):13388. doi: 10.1038/s41598-022-17064-8. Sci Rep. 2022. PMID: 35927412 Free PMC article.
References
-
- Pharmacopoeia B. 2016. Monograph on sulfacetamide sodium. Electronic version. London, UK: Her Majesty's Stationary Office.
-
- Lotfy H, Monir HH, El-Aleem A, El-Azizel-Bayoumi A. 2012. Novel spectrophotometric methods for the determination of fluconazole in the presence of its oxidative degradation product. J. Chil. Chem. Soc. 57, 1447-1455. (10.4067/S0717-97072012000400023) - DOI
-
- Aboul-Enein HY, Göğer NG, Türkalp A. 2002. Quantitative determination of fluconazole in syrups by first order derivative spectrophotometry. Anal. Lett. 35, 1193-1204. (10.1081/AL-120005972) - DOI
-
- Naveed S. 2015. UV spectrophotometric assay method for the determination of fluconazole capsules. OALib. J. 2, 1. (10.4236/oalib.1101468) - DOI
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

