An Evaluation of the Efficacy, Safety, and Tolerability of Abhraloha Compared With Oral Ferrous Ascorbate on Iron Deficiency Anemia in Women: A Randomized Controlled, Parallel-Group, Assessor-Blind Clinical Trial
- PMID: 33972905
- PMCID: PMC8104902
- DOI: 10.7759/cureus.14348
An Evaluation of the Efficacy, Safety, and Tolerability of Abhraloha Compared With Oral Ferrous Ascorbate on Iron Deficiency Anemia in Women: A Randomized Controlled, Parallel-Group, Assessor-Blind Clinical Trial
Abstract
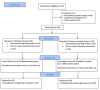
Background and objective Iron deficiency anemia (IDA) is a common condition in women for which ferrous ascorbate (FA) is often prescribed, which can lead to multiple side effects. Abhraloha is an Ayurvedic medicine that has been used for decades in India to treat IDA. In this study, we aimed to evaluate the efficacy and safety of Abhraloha with regard to change in hemoglobin (Hb) levels as compared to the standard treatment using FA in participants with IDA. Materials and methods We conducted a single-center, pragmatic, prospective, randomized, active-controlled, two-arm, parallel-group, assessor-blind study to evaluate the efficacy and safety of Abhraloha with regard to change in Hb levels as compared to the standard treatment using FA in participants suffering from IDA. The eligible participants were randomized and were advised to take either Abhraloha (two tablets twice a day) or FA (one tablet twice a day) for eight weeks; they were asked to follow up after 14 days for re-evaluation. On visit 1 and during the study period, the physician assessed the participants on the Pandurog scale and subjective variables. Descriptive statistics were used with unpaired T-test/Mann-Whitney U test for comparison between the groups. The Wilcoxon signed-rank test was used for within-group analysis, and the chi-square test/Fisher's exact test was employed for categorical data. Results Based on our findings, Abhraloha tablets significantly increased all the variables including the Pandurog scale after eight weeks of treatment. Abhraloha reduced total iron-binding capacity (TIBC) and peripheral smear lymphocyte (PSL), which is consistent with an improvement in IDA. There was a statistically significant increase in Hb, red blood cell (RBC) count, packed cell volume (PCV), mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) in the Abhraloha group as compared with the FA group at eight weeks. The Abhraloha group also exhibited a statistically significant improvement in all the subjective variables. Abhraloha was found to be safe and well-tolerated among the participants. Conclusions Abhraloha possesses hematinic activity and it improves all the blood indices. It is associated with significantly fewer adverse effects compared to oral iron therapy, which proves that it can be safely used for the treatment of IDA.
Keywords: iron deficiency anaemia (ida). anaemia of chronic disease(acd); iron profile; iron tablets.
Copyright © 2021, Gajbhiye et al.
Conflict of interest statement
Mr. Mukesh Chawda is an employee of Shri Dhootpapeshwar Limited (sponsor of the study).
References
-
- Government of India: Ministry of Health & Family Welfare - National Health Policy 2017. [Nov;2020 ];NATIONAL HEALTH POLICY, 2017 2017. https://www.nhp.gov.in/nhpfiles/national_health_policy_2017.pdf 2017
-
- The burden of iron-deficiency anaemia among women in India: how have iron and folic acid interventions fared? Rai RK, Fawzi WW, Barik A, Chowdhury A. WHO South East Asia J Public Health. 2018;7:18–23. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources