This is a preprint.
Mouse Adapted SARS-CoV-2 protects animals from lethal SARS-CoV challenge
- PMID: 33972939
- PMCID: PMC8109199
- DOI: 10.1101/2021.05.03.442357
Mouse Adapted SARS-CoV-2 protects animals from lethal SARS-CoV challenge
Update in
-
Mouse-adapted SARS-CoV-2 protects animals from lethal SARS-CoV challenge.PLoS Biol. 2021 Nov 4;19(11):e3001284. doi: 10.1371/journal.pbio.3001284. eCollection 2021 Nov. PLoS Biol. 2021. PMID: 34735434 Free PMC article.
Abstract
The emergence of SARS-CoV-2 has resulted in a worldwide pandemic causing significant damage to public health and the economy. Efforts to understand the mechanisms of COVID-19 disease have been hampered by the lack of robust mouse models. To overcome this barrier, we utilized a reverse genetic system to generate a mouse-adapted strain of SARS-CoV-2. Incorporating key mutations found in SARSCoV-2 variants, this model recapitulates critical elements of human infection including viral replication in the lung, immune cell infiltration, and significant in vivo disease. Importantly, mouse-adaptation of SARS-CoV-2 does not impair replication in human airway cells and maintains antigenicity similar to human SARS-CoV-2 strains. Utilizing this model, we demonstrate that SARS-CoV-2 infected mice are protected from lethal challenge with the original SARS-CoV, suggesting immunity from heterologous CoV strains. Together, the results highlight the utility of this mouse model for further study of SARS-CoV-2 infection and disease.
Conflict of interest statement
Competing interests
XX, P-YS, and VDM have filed a patent on the reverse genetic system and reporter SARS-CoV-2. Other authors declare no competing interests.
Figures
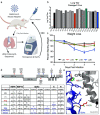
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
