Long-term and pathological outcomes of low- and intermediate-risk prostate cancer after radical prostatectomy: implications for active surveillance
- PMID: 33973043
- PMCID: PMC8521579
- DOI: 10.1007/s00345-021-03717-2
Long-term and pathological outcomes of low- and intermediate-risk prostate cancer after radical prostatectomy: implications for active surveillance
Abstract
Purpose: The safety of active surveillance (AS) in favorable intermediate-risk (FIR) prostate cancer (PCa) remains uncertain. To provide guidance on clinical decision-making, we examined long-term and pathological outcomes of low-risk and intermediate-risk PCa patients after radical prostatectomy (RP).
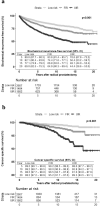
Methods: The study involved 5693 patients diagnosed between 1994 and 2019 with low-risk, FIR, and unfavorable intermediate-risk (UIR) PCa (stratification according to the AUA guidelines) who underwent RP. Pathological outcomes were compared, and Kaplan-Meier analysis determined biochemical recurrence-free survival (BRFS) and cancer-specific survival (CSS) at 5, 10, 15, and 20 years. Multiple Cox regression was used to simultaneously control for relevant confounders.
Results: Those at FIR had higher rates of upgrading and upstaging (12.8% vs. 7.2%, p < 0.001; 19.8% vs. 12.0%, p < 0.001) as well as pathological tumor and node stage (≥ pT3a: 18.8% vs. 11.6%, p < 0.001; pN1: 2.7% vs. 0.8%, p > 0.001) compared to patients at low risk. The 20-year BRFS was 69%, 65%, and 44% and the 20-year CSS was 98%, 95%, and 89% in low-risk, FIR, and UIR patients. On multiple Cox regression, FIR was not associated with a worse BRFS (HR 1.07, CI 0.87-1.32), UIR was associated with a worse BRFS (HR 1.49, CI 1.20-1.85).
Conclusion: Patients at FIR had only slightly worse pathological and long-term outcomes compared to patients at low risk, whereas the difference compared to patients at UIR was large. This emphasizes AS in these patients as a possible treatment strategy in well-counseled patients.
Keywords: Active surveillance; Favorable intermediate risk; Oncological outcome; Prostate cancer; Radical prostatectomy.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Comment in
-
Active surveillance for intermediate risk prostate cancer could be risky for the patient.World J Urol. 2022 Apr;40(4):1079-1080. doi: 10.1007/s00345-021-03767-6. Epub 2021 Jul 1. World J Urol. 2022. PMID: 34212236 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous