ACE2-based decoy receptors for SARS coronavirus 2
- PMID: 33973262
- PMCID: PMC8242511
- DOI: 10.1002/prot.26140
ACE2-based decoy receptors for SARS coronavirus 2
Abstract
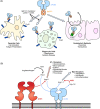
SARS coronavirus 2 is neutralized by proteins that block receptor-binding sites on spikes that project from the viral envelope. In particular, substantial research investment has advanced monoclonal antibody therapies to the clinic where they have shown partial efficacy in reducing viral burden and hospitalization. An alternative is to use the host entry receptor, angiotensin-converting enzyme 2 (ACE2), as a soluble decoy that broadly blocks SARS-associated coronaviruses with limited potential for viral escape. Here, we summarize efforts to engineer higher affinity variants of soluble ACE2 that rival the potency of affinity-matured antibodies. Strategies have also been used to increase the valency of ACE2 decoys for avid spike interactions and to improve pharmacokinetics via IgG fusions. Finally, the intrinsic catalytic activity of ACE2 for the turnover of the vasoconstrictor angiotensin II may directly address COVID-19 symptoms and protect against lung and cardiovascular injury, conferring dual mechanisms of action unachievable by monoclonal antibodies. Soluble ACE2 derivatives therefore have the potential to be next generation therapeutics for addressing the immediate needs of the current pandemic and possible future outbreaks.
Keywords: ACE2; COVID-19; SARS coronavirus 2; avidity; decoy receptor; protein engineering.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
The University of Illinois has filed a provisional patent for engineered decoy receptors with Erik Procko as an inventor. Erik Procko is a co‐founder of Orthogonal Biologics, Inc.
Figures



References
-
- Adam DC, Wu P, Wong JY, et al. Clustering and superspreading potential of SARS‐CoV‐2 infections in Hong Kong. Nat Med. 2020;26:1714‐1719. - PubMed
-
- He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID‐19. Nat Med. 2020;26:672‐675. - PubMed
-
- Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nat Med. 2020;26:1200‐1204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

