FDA Approval Summary: Nivolumab Plus Ipilimumab for the Treatment of Patients with Hepatocellular Carcinoma Previously Treated with Sorafenib
- PMID: 33973307
- PMCID: PMC8417871
- DOI: 10.1002/onco.13819
FDA Approval Summary: Nivolumab Plus Ipilimumab for the Treatment of Patients with Hepatocellular Carcinoma Previously Treated with Sorafenib
Abstract
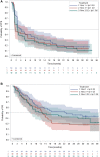
On March 10, 2020, the U.S. Food and Drug Administration (FDA) granted accelerated approval to nivolumab in combination with ipilimumab for the treatment of patients with hepatocellular carcinoma (HCC) previously treated with sorafenib. The recommended approved dosage was nivolumab 1 mg/kg i.v. plus ipilimumab 3 mg/kg i.v. every 3 weeks for four cycles, followed by nivolumab 240 mg i.v. every 2 weeks. The approval was based on data from cohort 4 of CheckMate 040, which randomized patients with advanced unresectable or metastatic HCC previously treated with or who were intolerant to sorafenib to receive one of three different dosing regimens of nivolumab in combination with ipilimumab. Investigator-assessed overall response rate (ORR) was the primary endpoint, and ORR assessed by blinded independent central review (BICR) was an exploratory endpoint. BICR-assessed ORR and duration of response (DoR) form the primary basis of the FDA's regulatory decision, and BICR-assessed ORR was comparable in all three arms at 31%-32% with 95% confidence interval [CI] 18%-47%. The DoR ranged from 17.5 to 22.2 months across the three arms, with overlapping 95% CIs. Adverse events (AEs) were generally consistent with the known AE profiles of nivolumab and ipilimumab, and no new safety events were identified. This article summarizes the FDA review of the data supporting the approval of nivolumab and ipilimumab for the treatment of HCC. IMPLICATIONS FOR PRACTICE: Nivolumab and ipilimumab combination therapy is another option for patients with advanced hepatocellular carcinoma who experience radiographic progression during or after sorafenib or sorafenib intolerance. No new toxicities were identified, but, as expected, increased toxicity was observed with the addition of ipilimumab to nivolumab as compared with nivolumab alone, which is also approved for the same indication. Whether to administer nivolumab as a single agent or in combination with ipilimumab is expected to be a joint decision between the oncologist and patient, taking into consideration the potential for a higher likelihood of response and the potentially higher rate of toxicity with the combination.
Keywords: CheckMate 040; Hepatocellular carcinoma; Ipilimumab; Nivolumab.
Published 2021. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
Figures
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2020. CA Cancer J Clin 2020;70:7–30. - PubMed
-
- Ha J, Yan M, Aguilar M et al. Race/ethnicity‐specific disparities in cancer incidence, burden of disease, and overall survival among patients with hepatocellular carcinoma in the United States. Cancer 2016;122:2512–2523. - PubMed
-
- Bruix J, Qin S, Merle P et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2017;389:56–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

