Stimulated saprotrophic fungi in arable soil extend their activity to the rhizosphere and root microbiomes of crop seedlings
- PMID: 33973345
- PMCID: PMC8596668
- DOI: 10.1111/1462-2920.15563
Stimulated saprotrophic fungi in arable soil extend their activity to the rhizosphere and root microbiomes of crop seedlings
Abstract
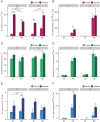
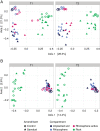
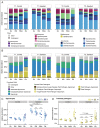
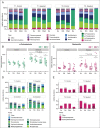
Saprotrophic fungi play an important role in ecosystem functioning and plant performance, but their abundance in intensively managed arable soils is low. Saprotrophic fungal biomass in arable soils can be enhanced with amendments of cellulose-rich materials. Here, we examined if sawdust-stimulated saprotrophic fungi extend their activity to the rhizosphere of crop seedlings and influence the composition and activity of other rhizosphere and root inhabitants. After growing carrot seedlings in sawdust-amended arable soil, we determined fungal and bacterial biomass and community structure in roots, rhizosphere and soil. Utilization of root exudates was assessed by stable isotope probing (SIP) following 13 CO2 -pulse-labelling of seedlings. This was combined with analysis of lipid fatty acids (PLFA/NLFA-SIP) and nucleic acids (DNA-SIP). Sawdust-stimulated Sordariomycetes colonized the seedling's rhizosphere and roots and actively consumed root exudates. This did not reduce the abundance and activity of bacteria, yet higher proportions of α-Proteobacteria and Bacteroidia were seen. Biomass and activity of mycorrhizal fungi increased with sawdust amendments, whereas exudate consumption and root colonization by functional groups containing plant pathogens did not change. Sawdust amendment of arable soil enhanced abundance and exudate-consuming activity of saprotrophic fungi in the rhizosphere of crop seedlings and promoted potential beneficial microbial groups in root-associated microbiomes.
© 2021 The Authors. Environmental Microbiology published by Society for Applied Microbiology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Shifts in rhizosphere fungal community during secondary succession following abandonment from agriculture.ISME J. 2017 Oct;11(10):2294-2304. doi: 10.1038/ismej.2017.90. Epub 2017 Jun 6. ISME J. 2017. PMID: 28585935 Free PMC article.
-
Evaluation of Phenolic Root Exudates as Stimulants of Saptrophic Fungi in the Rhizosphere.Front Microbiol. 2021 Apr 14;12:644046. doi: 10.3389/fmicb.2021.644046. eCollection 2021. Front Microbiol. 2021. PMID: 33936001 Free PMC article.
-
Fungal-Bacterial Cooccurrence Patterns Differ between Arbuscular Mycorrhizal Fungi and Nonmycorrhizal Fungi across Soil Niches.mBio. 2021 Apr 20;12(2):e03509-20. doi: 10.1128/mBio.03509-20. mBio. 2021. PMID: 33879589 Free PMC article.
-
Root exudates: from plant to rhizosphere and beyond.Plant Cell Rep. 2020 Jan;39(1):3-17. doi: 10.1007/s00299-019-02447-5. Epub 2019 Jul 25. Plant Cell Rep. 2020. PMID: 31346716 Review.
-
Global magnitude of rhizosphere effects on soil microbial communities and carbon cycling in natural terrestrial ecosystems.Sci Total Environ. 2023 Jan 15;856(Pt 1):158961. doi: 10.1016/j.scitotenv.2022.158961. Epub 2022 Sep 22. Sci Total Environ. 2023. PMID: 36155049 Review.
Cited by
-
The plant protection preparation GZM improves crop immunity, yield, and quality.iScience. 2023 May 5;26(6):106819. doi: 10.1016/j.isci.2023.106819. eCollection 2023 Jun 16. iScience. 2023. PMID: 37250797 Free PMC article.
-
Comparative Analysis of Nano-Bactericides and Thiodiazole-Copper on Tomato Rhizosphere Microbiome.Microorganisms. 2025 Jun 7;13(6):1327. doi: 10.3390/microorganisms13061327. Microorganisms. 2025. PMID: 40572215 Free PMC article.
-
Tipping the plant-microbe competition for nitrogen in agricultural soils.iScience. 2024 Sep 18;27(10):110973. doi: 10.1016/j.isci.2024.110973. eCollection 2024 Oct 18. iScience. 2024. PMID: 39391734 Free PMC article. Review.
-
Impacts of coniferous bark-derived organic soil amendments on microbial communities in arable soil - a microcosm study.FEMS Microbiol Ecol. 2023 Feb 28;99(3):fiad012. doi: 10.1093/femsec/fiad012. FEMS Microbiol Ecol. 2023. PMID: 36725205 Free PMC article.
-
Deciphering the core seed endo-bacteriome of the highland barley in Tibet plateau.Front Plant Sci. 2022 Oct 28;13:1041504. doi: 10.3389/fpls.2022.1041504. eCollection 2022. Front Plant Sci. 2022. PMID: 36388601 Free PMC article.
References
-
- Abarenkov, K. , Henrik Nilsson, R. , Larsson, K.‐H. , Alexander, I.J. , Eberhardt, U. , Erland, S. , et al. (2010) The UNITE database for molecular identification of fungi – recent updates and future perspectives. New Phytol 186: 281–285. - PubMed
-
- Anderson, M.J. , and Walsh, D.C.I. (2013) PERMANOVA, ANOSIM, and the mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecol Monogr 83: 557–574.
-
- Badri, D.V. , Chaparro, J.M. , Zhang, R. , Shen, Q. , and Vivanco, J.M. (2013) Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic‐related compounds predominantly modulate the soil microbiome. J Biol Chem 288: 30503. - PMC - PubMed
-
- Ballhausen, M.‐B. , and de Boer, W. (2016) The sapro‐rhizosphere: carbon flow from saprotrophic fungi into fungus‐feeding bacteria. Soil Biol Biochem 102: 14–17.
-
- Beare, M.H. , Hu, S. , Coleman, D.C. , and Hendrix, P.F. (1997) Influences of mycelial fungi on soil aggregation and organic matter storage in conventional and no‐tillage soils. Appl Soil Ecol 5: 211–219.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

