Limonin as a Starting Point for the Construction of Compounds with High Scaffold Diversity
- PMID: 33973348
- PMCID: PMC8260459
- DOI: 10.1002/anie.202104228
Limonin as a Starting Point for the Construction of Compounds with High Scaffold Diversity
Abstract
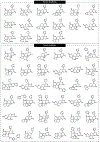
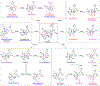
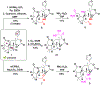
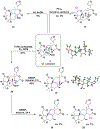
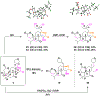
Structurally complex natural products have been a fruitful source for the discovery and development of new drugs. In an effort to construct a compound collection populated by architecturally complex members with unique scaffolds, we have used the natural product limonin as a starting point. Limonin is an abundant triterpenoid natural product and, through alteration of its heptacyclic core ring system using short synthetic sequences, a collection of 98 compounds was created, including multiple members with novel ring systems. The reactions leveraged in the construction of these compounds include novel ring cleavage, rearrangements, and cyclizations, and this work is highlighted by the discovery of a novel B-ring cleavage reaction, a unique B/C-ring rearrangement, an atypical D-ring cyclization, among others. Computational analysis shows that 52 different scaffolds/ring systems were produced during the course of this work, of which 36 are unprecedented. Phenotypic screening and structure-activity relationships identified compounds with activity against a panel of cancer cell lines.
Keywords: anticancer agents; drug discovery; limonin; natural products; scaffold diversity.
© 2021 Wiley-VCH GmbH.
Figures

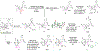
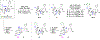
Similar articles
-
Chemical Derivatization Leads to the Discovery Of Novel Analogs of Azotochelin, a Natural Siderophore, as Promising Anticancer Agents.ChemMedChem. 2024 Jul 2;19(13):e202300715. doi: 10.1002/cmdc.202300715. Epub 2024 May 14. ChemMedChem. 2024. PMID: 38598189
-
[Progress in research of the structural optimization of natural product-like Garcinia caged xanthones].Yao Xue Xue Bao. 2014 Mar;49(3):293-302. Yao Xue Xue Bao. 2014. PMID: 24961098 Review. Chinese.
-
Synthesis and cytotoxicity of novel imidazo[4,5-d]azepine compounds derived from marine natural product ceratamine A.Bioorg Med Chem Lett. 2018 Mar 1;28(5):866-868. doi: 10.1016/j.bmcl.2018.02.004. Epub 2018 Feb 9. Bioorg Med Chem Lett. 2018. PMID: 29433924
-
A multicomponent macrocyclization strategy to natural product-like cyclic lipopeptides: synthesis and anticancer evaluation of surfactin and mycosubtilin analogues.Org Biomol Chem. 2017 May 3;15(17):3628-3637. doi: 10.1039/c7ob00459a. Org Biomol Chem. 2017. PMID: 28406518
-
Applications of innovative synthetic strategies in anticancer drug discovery: The driving force of new chemical reactions.Bioorg Med Chem Lett. 2025 Apr 15;119:130096. doi: 10.1016/j.bmcl.2025.130096. Epub 2025 Jan 9. Bioorg Med Chem Lett. 2025. PMID: 39798856 Review.
Cited by
-
Raptinal: a powerful tool for rapid induction of apoptotic cell death.Cell Death Discov. 2024 Aug 21;10(1):371. doi: 10.1038/s41420-024-02120-1. Cell Death Discov. 2024. PMID: 39164225 Free PMC article. Review.
-
Post-Cyclization Skeletal Rearrangements in Plant Triterpenoid Biosynthesis by a Pair of Branchpoint Isomerases.J Am Chem Soc. 2023 Mar 8;145(9):5083-5091. doi: 10.1021/jacs.2c10838. Epub 2023 Feb 23. J Am Chem Soc. 2023. PMID: 36821810 Free PMC article.
-
Target-Agnostic P-Glycoprotein Assessment Yields Strategies to Evade Efflux, Leading to a BRAF Inhibitor with Intracranial Efficacy.J Am Chem Soc. 2022 Jul 13;144(27):12367-12380. doi: 10.1021/jacs.2c03944. Epub 2022 Jun 27. J Am Chem Soc. 2022. PMID: 35759775 Free PMC article.
-
Insights into the Mechanism of Action of the Degraded Limonoid Prieurianin.Int J Mol Sci. 2024 Mar 22;25(7):3597. doi: 10.3390/ijms25073597. Int J Mol Sci. 2024. PMID: 38612409 Free PMC article. Review.
-
Enhancing structural diversity through chemical engineering of Ambrosia tenuifolia extract for novel anti-glioblastoma compounds.Sci Rep. 2024 Jun 20;14(1):14229. doi: 10.1038/s41598-024-63639-y. Sci Rep. 2024. PMID: 38902325 Free PMC article.
References
-
- Brown DG, Bostrom J, J. Med. Chem 2018, 61, 9442–9468. - PubMed
-
- Macarron R, Banks M, Bojanic D, Burns D, Cirovic D, Garyantes T, Green D, Hertzberg R, Janzen W, Paslay J, Schopfer U, Sittampalam G, Nat. Rev. Drug Discov 2011, 10, 188–195. - PubMed
-
- Lovering F, Bikker J, Humblet C, J. Med. Chem 2009, 52, 6752–6756. - PubMed
-
- Tajabadi FM, Campitelli MR, Quinn RJ, Springer Sci. Rev 2013, 1, 141–151.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

