Astragaloside alleviates alcoholic fatty liver disease by suppressing oxidative stress
- PMID: 33973356
- PMCID: PMC11896517
- DOI: 10.1002/kjm2.12390
Astragaloside alleviates alcoholic fatty liver disease by suppressing oxidative stress
Abstract
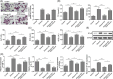
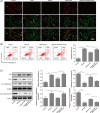
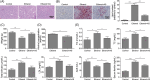
Alcoholic fatty liver disease (AFLD) is the most common liver disease and can progress to fatal liver cirrhosis and carcinoma, affecting millions of patients worldwide. The functions of astragaloside on the cardiovascular system have been elucidated. However, its role in AFLD is unclear. Ethanol-treated AML-12 cells were used as a cell model of alcoholic fatty liver. Real-time quantitative reverse transcription-PCR and Western blotting detected genes and proteins expressions. Reactive oxygen species (ROS), triglyceride, total cholesterol, low-density lipoprotein, albumin, ferritin, bilirubin, superoxide dismutase, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were examined using commercial kits. Lipid accumulation was assessed by Oil red O staining. MTT and flow cytometry measured cell viability and apoptosis. JC-1 was used to analyze mitochondrial membrane potential. A rat model of AFLD was established by treating rats with ethanol. Astragaloside suppressed ethanol-induced lipid accumulation, oxidative stress, and the production of AST and ALT in AML-12 cells. Ethanol induced TNF-α and reduced IL-10 expression, which were reversed by astragaloside. Ethanol promoted Bax expression and cytochrome C release and inhibited Bcl-2 and ATP expression. Astragaloside hampered these apoptosis effects in AML-12 cells. Impaired mitochondrial membrane potential was recovered by astragaloside. However, all these astragaloside-mediated beneficial effects were abolished by the ROS inducer pyocyanin. Ethanol-induced activation of NF-κB signaling was suppressed by astragaloside in vitro and in vivo, suggesting that astragaloside inhibited oxidative stress by suppressing the activation of NF-κB signaling, thus improving liver function and alleviating AFLD in rats. Our study elucidates the pharmacological mechanism of astragaloside and provides potential therapeutic strategies for AFLD.
Keywords: alcoholic fatty liver disease; astragaloside; lipid accumulation; oxidative stress.
© 2021 The Authors. The Kaohsiung Journal of Medical Sciences published by John Wiley & Sons Australia on behalf of Kaohsiung Medical University.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



References
-
- Rakha EA, Adamson L, Bell E, Neal K, Ryder SD, Kaye PV, et al. Portal inflammation is associated with advanced histological changes in alcoholic and non‐alcoholic fatty liver disease. J Clin Pathol. 2010;63(9):790–5. - PubMed
-
- Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol‐induced liver injury. Arch Toxicol. 2009;83(6):519–48. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

