Adherence to event-driven HIV PrEP among men who have sex with men in Amsterdam, the Netherlands: analysis based on online diary data, 3-monthly questionnaires and intracellular TFV-DP
- PMID: 33973373
- PMCID: PMC8110892
- DOI: 10.1002/jia2.25708
Adherence to event-driven HIV PrEP among men who have sex with men in Amsterdam, the Netherlands: analysis based on online diary data, 3-monthly questionnaires and intracellular TFV-DP
Abstract
Introduction: Event-driven pre-exposure prophylaxis (edPrEP) with oral tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) is highly effective for preventing HIV acquisition in men who have sex with men (MSM) and is preferred over daily PrEP by some MSM. However, it is largely unknown how well MSM adhere to edPrEP. We then aimed to assess PrEP protection during CAS among MSM using edPrEP and participating in the Amsterdam PrEP demonstration project (AMPrEP).
Methods: We analysed data from participants enrolled in AMPrEP who were taking edPrEP. We measured adherence through (1) a mobile application in which sexual behaviour and PrEP-use were recorded daily, (2) three-monthly self-completed questionnaires and (3) dried blood spot (DBS) samples collected around six, twelve and twenty-four months after PrEP initiation. We assessed the proportion of days with condomless anal sex (CAS) acts that were protected by PrEP, per partner type (i.e. steady partners, known casual partners, unknown casual partners), and the proportion of three-month periods during which PrEP was correctly used. Intracellular TFV-diphosphate (TFV-DP) concentrations were determined from DBS. Good adherence was defined as at least one tablet before and one tablet within 48 hours after a CAS act.
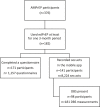
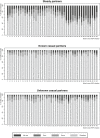
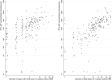
Results: Between 11 September 2015 and 6 October 2019, 182 of 376 MSM (48.4%) used edPrEP for at least one three-month period. Of the 8224 CAS days that were reported in the app during edPrEP-use, we observed good protection for most CAS days involving steady partners (n = 1625/2455, 66.9%), known casual partners (n = 3216/3472, 92.6%) and unknown casual partners (n = 2074/2297, 90.3%). Men reported consistently correct PrEP-use in 851 (81.4%) of the 1046 three-month periods of edPrEP-use. The median TFV-DP concentration was 591 fmol/sample (interquartile range = 270 to 896).
Conclusions: Adherence to edPrEP was high as determined from the online app and questionnaire. DBS measurements were consistent with two to three tablets per week on average.
Keywords: HIV; HIV prevention and control; On-demand PrEP; Pre-exposure prophylaxis; medication adherence; men who have sex with men.
© 2021 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Figures
Similar articles
-
Determinants of adherence to daily PrEP measured as intracellular tenofovir diphosphate concentrations over 24 months of follow-up among men who have sex with men.Sex Transm Infect. 2023 Aug;99(5):303-310. doi: 10.1136/sextrans-2022-055499. Epub 2022 Sep 5. Sex Transm Infect. 2023. PMID: 37258273 Free PMC article.
-
Youth-friendly services and a mobile phone application to promote adherence to pre-exposure prophylaxis among adolescent men who have sex with men and transgender women at-risk for HIV in Thailand: a randomized control trial.J Int AIDS Soc. 2020 Sep;23 Suppl 5(Suppl 5):e25564. doi: 10.1002/jia2.25564. J Int AIDS Soc. 2020. PMID: 32869511 Free PMC article. Clinical Trial.
-
High pre-exposure prophylaxis uptake and early adherence among men who have sex with men and transgender women at risk for HIV Infection: the PrEP Brasil demonstration project.J Int AIDS Soc. 2017 Apr 6;20(1):21472. doi: 10.7448/IAS.20.1.21472. J Int AIDS Soc. 2017. PMID: 28418232 Free PMC article.
-
Pre-exposure Prophylaxis (PrEP) for HIV Prevention Among Men Who Have Sex with Men (MSM): A Scoping Review on PrEP Service Delivery and Programming.AIDS Behav. 2020 Nov;24(11):3056-3070. doi: 10.1007/s10461-020-02855-9. AIDS Behav. 2020. PMID: 32274670 Free PMC article.
-
The Italian PrEPventHIV challenge: a scoping systematic review on HIV pre-exposure prophylaxis monitoring in Italy.Acta Biomed. 2022 Jul 1;93(3):e2022164. doi: 10.23750/abm.v93i3.12766. Acta Biomed. 2022. PMID: 35775760 Free PMC article.
Cited by
-
HIV-1-infection in a man who has sex with men despite self-reported excellent adherence to pre-exposure prophylaxis, the Netherlands, August 2021: be alert to emtricitabine/tenofovir-resistant strain transmission.Euro Surveill. 2022 Apr;27(14):2200275. doi: 10.2807/1560-7917.ES.2022.27.14.2200275. Euro Surveill. 2022. PMID: 35393931 Free PMC article.
-
The Disparities of PrEP Adherence Among Men Who Have Sex With Men Between the Global South and the Global North: An Updated Determinantal Global Meta-Analysis.J Acquir Immune Defic Syndr. 2025 May 1;99(1):1-8. doi: 10.1097/QAI.0000000000003635. J Acquir Immune Defic Syndr. 2025. PMID: 39878582 Free PMC article.
-
A Novel Algorithm to Improve PrEP Adherence Monitoring Using Dried Blood Spots.Clin Pharmacol Ther. 2023 Apr;113(4):896-903. doi: 10.1002/cpt.2845. Epub 2023 Jan 31. Clin Pharmacol Ther. 2023. PMID: 36622798 Free PMC article.
-
Determinants of PrEP Uptake, Intention and Awareness in the Netherlands: A Socio-Spatial Analysis.Int J Environ Res Public Health. 2022 Jul 20;19(14):8829. doi: 10.3390/ijerph19148829. Int J Environ Res Public Health. 2022. PMID: 35886681 Free PMC article.
-
Trajectories of PrEP use among men who have sex with men: a pooled analysis of two prospective, observational cohort studies.J Int AIDS Soc. 2023 Jul;26(7):e26133. doi: 10.1002/jia2.26133. J Int AIDS Soc. 2023. PMID: 37501262 Free PMC article.
References
-
- Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On‐demand preexposure prophylaxis in men at high risk for HIV‐1 infection. N Engl J Med. 2015;373(23):2237–46. - PubMed
-
- WHO . Guidelines on when to start antiretroviral therapy and on pre‐exposure prophylaxis for HIV. Geneva: World Health Organization; 2015. - PubMed
-
- Antoni G, Tremblay C, Delaugerre C, Charreau I, Cua E, Rojas Castro D, et al. On‐demand pre‐exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post‐hoc analysis of the ANRS IPERGAY trial. The Lancet HIV. 2020;7(2):e113–20. - PubMed
-
- Molina J‐M, Charreau I, Spire B, Cotte L, Chas J, Capitant C, et al. Efficacy, safety, and effect on sexual behaviour of on‐demand pre‐exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV. 2017;4(9):e402–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

