Hypertrophic Preconditioning Attenuates Myocardial Ischaemia-Reperfusion Injury by Modulating SIRT3-SOD2-mROS-Dependent Autophagy
- PMID: 33973685
- PMCID: PMC8249780
- DOI: 10.1111/cpr.13051
Hypertrophic Preconditioning Attenuates Myocardial Ischaemia-Reperfusion Injury by Modulating SIRT3-SOD2-mROS-Dependent Autophagy
Abstract
Background: Ischaemic preconditioning elicited by brief periods of coronary occlusion and reperfusion protects the heart from a subsequent prolonged ischaemic insult. Here, we test the hypothesis that short-term non-ischaemic stimulation of hypertrophy renders the heart resistant to subsequent ischaemic injury.
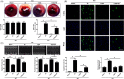
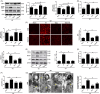
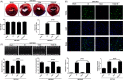
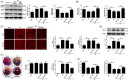
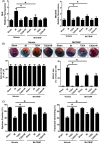
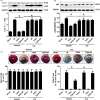
Methods and results: Transient transverse aortic constriction (TAC) was performed for 3 days in mice and then withdrawn for 4 days by aortic debanding, followed by subsequent exposure to myocardial ischaemia-reperfusion (I/R) injury. Following I/R injury, myocardial infarct size and apoptosis were significantly decreased, and cardiac dysfunction was markedly improved in the TAC preconditioning group compared with the control group. Mechanistically, TAC preconditioning markedly suppressed I/R-induced autophagy and preserved autophagic flux by deacetylating SOD2 via a SIRT3-dependent mechanism. Moreover, treatment with an adenovirus encoding SIRT3 partially mimicked the effects of hypertrophic preconditioning, whereas genetic ablation of SIRT3 in mice blocked the cardioprotective effects of hypertrophic preconditioning. Furthermore, in vivo lentiviral-mediated knockdown of Beclin 1 in the myocardium ameliorated the I/R-induced impairment of autophagic flux and was associated with a reduction in cell death, whereas treatment with a lentivirus encoding Beclin 1 abolished the cardioprotective effect of TAC preconditioning.
Conclusions: The present study identifies TAC preconditioning as a novel strategy for induction of an endogenous self-defensive and cardioprotective mechanism against cardiac injury. Specifically, TAC preconditioning reduced myocardial autophagic cell death in a SIRT3/SOD2 pathway-dependent manner.
Keywords: SIRT3; autophagy; hypertrophic preconditioning; ischaemia-reperfusion injury.
© 2021 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
None.
Figures


References
-
- Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. 2017;376(21):2053‐2064. - PubMed
-
- Cung T‐T, Morel O, Cayla G, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373(11):1021‐1031. - PubMed
-
- Cabrera‐Fuentes HA, Aragones J, Bernhagen J, et al. From basic mechanisms to clinical applications in heart protection, new players in cardiovascular diseases and cardiac theranostics: meeting report from the third international symposium on "New frontiers in cardiovascular research". Basic Res Cardiol. 2016;111(6):69. - PMC - PubMed
-
- Heusch G, Rassaf T. Time to give up on cardioprotection? A critical appraisal of clinical studies on ischemic pre‐, post‐, and remote conditioning. Circ Res. 2016;119(5):676‐695. - PubMed
-
- Zhang L, Wang K, Lei Y, Li Q, Nice EC, Huang C. Redox signaling: potential arbitrator of autophagy and apoptosis in therapeutic response. Free Radic Biol Med. 2015;89:452‐465. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

