Connectivity within regions characterizes epilepsy duration and treatment outcome
- PMID: 33973688
- PMCID: PMC8288103
- DOI: 10.1002/hbm.25464
Connectivity within regions characterizes epilepsy duration and treatment outcome
Abstract
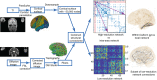
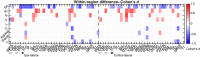
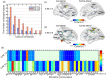
Finding clear connectome biomarkers for temporal lobe epilepsy (TLE) patients, in particular at early disease stages, remains a challenge. Currently, the whole-brain structural connectomes are analyzed based on coarse parcellations (up to 1,000 nodes). However, such global parcellation-based connectomes may be unsuitable for detecting more localized changes in patients. Here, we use a high-resolution network (~50,000-nodes overall) to identify changes at the local level (within brain regions) and test its relation with duration and surgical outcome. Patients with TLE (n = 33) and age-, sex-matched healthy subjects (n = 36) underwent high-resolution (~50,000 nodes) structural network construction based on deterministic tracking of diffusion tensor imaging. Nodes were allocated to 68 cortical regions according to the Desikan-Killany atlas. The connectivity within regions was then used to predict surgical outcome. MRI processing, network reconstruction, and visualization of network changes were integrated into the NICARA (https://nicara.eu). Lower clustering coefficient and higher edge density were found for local connectivity within regions in patients, but were absent for the global network between regions (68 cortical regions). Local connectivity changes, in terms of the number of changed regions and the magnitude of changes, increased with disease duration. Local connectivity yielded a better surgical outcome prediction (Mean value: 95.39% accuracy, 92.76% sensitivity, and 100% specificity) than global connectivity. Connectivity within regions, compared to structural connectivity between brain regions, can be a more efficient biomarker for epilepsy assessment and surgery outcome prediction of medically intractable TLE.
Keywords: epilepsy duration; high-resolution structural network; network metrics; surgical outcome prediction; within brain region.
© 2021 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Ahmadi, M. E., Hagler, D. J., McDonald, C. R., Tecoma, E. S., Iragui, V. J., Dale, A. M., & Halgren, E. (2009). Side Matters: Diffusion Tensor Imaging Tractography in Left and Right Temporal Lobe Epilepsy. American Journal of Neuroradiology, 30(9), 1740–1747. 10.3174/ajnr.a1650. - DOI - PMC - PubMed
-
- Alarcon, G, Garcia Seoane, J. J. , Binnie, C. D. , Martin Miguel, M. C. , Juler, J. , Polkey, C. E. , … Ortiz Blasco, J. M. (1997). Origin and propagation of interictal discharges in the acute electrocorticogram. Implications for pathophysiology and surgical treatment of temporal lobe epilepsy. Brain, 120(12), 2259–2282. 10.1093/brain/120.12.2259. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

