Lower risk of hospitalization for heart failure, kidney disease and death with sodium-glucose co-transporter-2 inhibitors compared with dipeptidyl peptidase-4 inhibitors in type 2 diabetes regardless of prior cardiovascular or kidney disease: A retrospective cohort study in UK primary care
- PMID: 33973690
- PMCID: PMC8518855
- DOI: 10.1111/dom.14437
Lower risk of hospitalization for heart failure, kidney disease and death with sodium-glucose co-transporter-2 inhibitors compared with dipeptidyl peptidase-4 inhibitors in type 2 diabetes regardless of prior cardiovascular or kidney disease: A retrospective cohort study in UK primary care
Abstract
Aim: To assess if sodium-glucose co-transporter-2 inhibitors (SGLT2is) reduce the risk of all-cause mortality, cardiovascular death and hospitalization for heart failure (HF) or chronic kidney disease (CKD) to a greater extent than dipeptidyl peptidase-4 inhibitors (DPP4is) in people with type 2 diabetes (T2D) with or without established cardiovascular and/or renal disease (CVRD).
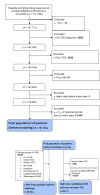
Methods: This retrospective cohort study propensity-matched 24 438 patients receiving an SGLT2i 1:1 to a patient receiving a DDP4i, stratified based on the presence of CVRD. The primary outcomes were the time to each of the following: all-cause mortality, cardiovascular death or hospitalization for HF, myocardial infarction, stroke and CKD.
Results: Overall, SGLT2is were associated with reductions in all-cause mortality, cardiovascular mortality, hospitalization for HF and hospitalization for CKD compared with DPP4is. In patients with no CVRD history, SGLT2is were associated with reductions in all-cause mortality (HR 0.71, 95% CI 0.57-0.88; P = .002), hospitalization for HF (HR 0.76, 95% CI 0.59-0.98; P = .035) and hospitalization for CKD (HR 0.75, 95% CI 0.63-0.88; P < .001). In patients with established cardiovascular disease (CVD) or at high risk, SGLT2is were associated with reductions in all-cause mortality (HR 0.69, 95% CI 0.59-0.82; P < .001), cardiovascular mortality (HR 0.76, 95% CI 0.62-0.95; P = .014), hospitalization for HF (HR 0.73, 95% CI 0.63-0.85; P < .001), hospitalization for stroke (HR 0.75, 95% CI 0.59-0.94; P = .013) and hospitalization for CKD (HR 0.49, 95% CI 0.43-0.54; P < .001).
Conclusion: There was consistency across subgroups and sensitivity analyses. SGLT2is were associated with a reduced risk of all-cause mortality and hospitalization for HF and CKD compared with DPP4-is, highlighting the need to introduce SGLT2is early in the management of patients with T2D.
Keywords: cardiovascular disease; clinical trial; dapagliflozin; diabetes complications; dipeptidyl peptidase-4 inhibitor; heart failure.
© 2021 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
KK has acted as a consultant, speaker or received grants for investigator‐initiated studies for AstraZeneca, Novartis, Novo Nordisk, Sanofi–Aventis, Lilly and Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin–Chemie AG/Menarini Group, Janssen and Napp. II has acted as an advisory board member, speaker or received grants for Eli Lilly, Novo Nordisk, Merck Sharp & Dohme, AstraZeneca, Abbot Diabetes Care, Sanofi and Boehringer. RZ, JBM, MF and TM are employed by AstraZeneca UK Ltd, a biopharmaceutical company that develops, manufactures and markets medicines in the cardiovascular, renal and metabolic disease area. AB has received research grants from AstraZeneca.
Figures

References
-
- Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27(8):1879‐1884. - PubMed
-
- Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG. Prevalence and risk factors formicroalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69(11):2057‐2063. - PubMed
-
- Thrainsdottir IS, Aspelund T, Thorgeirsson G, et al. The association between glucose abnormalities and heart failure in the population‐based Reykjavik study. Diabetes Care. 2005;28(3):612‐616. - PubMed
-
- Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis. 2019;62(4):298‐302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

