Virologic efficacy of tenofovir, lamivudine and dolutegravir as second-line antiretroviral therapy in adults failing a tenofovir-based first-line regimen
- PMID: 33973876
- PMCID: PMC7612028
- DOI: 10.1097/QAD.0000000000002936
Virologic efficacy of tenofovir, lamivudine and dolutegravir as second-line antiretroviral therapy in adults failing a tenofovir-based first-line regimen
Abstract
Objective: Recycling tenofovir and lamivudine/emtricitabine (XTC) with dolutegravir would provide a more tolerable, affordable, and scalable second-line regimen than dolutegravir with an optimized nucleoside reverse transcriptase inhibitor (NRTI) backbone. We evaluated efficacy of tenofovir/lamivudine/dolutegravir (TLD) in patients failing first-line tenofovir/XTC/efavirenz or nevirapine.
Design: Single arm, prospective, interventional study.
Setting: Two primary care clinics in Khayelitsha, South Africa.
Participants: Sixty adult patients with two viral loads greater than 1000 copies/ml.
Intervention: Participants were switched to TLD with additional dolutegravir (50 mg) for 2 weeks to overcome efavirenz induction.
Primary outcome: Proportion achieving viral load less than 50 copies/ml at week 24 using the FDA snapshot algorithm.
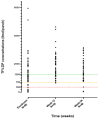
Results: Baseline median CD4+ cell count was 248 cells/μl, viral load 10 580 copies/ml and 48 of 54 (89%) had resistance (Stanford score ≥15) to one or both of tenofovir and XTC. No participants were lost to follow-up. At week 24, 51 of 60 [85%, 95% confidence interval (CI) 73-93%] were virologically suppressed, six had viral load 50-100 copies/ml, one had viral load 100-1000 copies/ml, one no viral load in window, and one switched because of tenofovir-related adverse event. No integrase mutations were detected in the one participant meeting criteria for resistance testing. Virological suppression was achieved by 29 of 35 (83%, 95% CI 66-93%) with resistance to tenofovir and XTC, 11 of 13 (85%, 95% CI 55-98%) with resistance to XTC, and six of six (100%, 95% CI 54-100%) with resistance to neither.
Conclusion: A high proportion of adults switching to second-line TLD achieved virologic suppression despite substantial baseline NRTI resistance and most not suppressed had low-level viraemia (≤100 copies/ml). This suggests recycling tenofovir and XTC with dolutegravir could provide an effective second-line option.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Figures

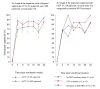
<350 fmol/punch (equivalent of men: <1.2 doses per week and women: <0.6 doses per week)
350-700 fmol/punch (men: 1.2 -3.2 doses per week and women: 0.6 -2.0 doses per week)
700-1250 fmol/punch (men: 3.2-6 doses per week and women: 2.0-5.3 doses per week)
1250 fmol/punch (men: >6 doses per week and women: >5.3 doses per week)
Comment in
-
Growing data for recycling tenofovir and lamivudine with dolutegravir as empiric second-line antiretroviral therapy in resource-limited settings.AIDS. 2021 Jul 15;35(9):1505-1507. doi: 10.1097/QAD.0000000000002958. AIDS. 2021. PMID: 34185718 No abstract available.
References
-
- Aboud M, Kaplan R, Lombaard J, Zhang F, Hidalgo JA, Mamedova E, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis. 2019;19(3):253–64. - PubMed
-
- The World Health Organization. Update of recommendations on first- and second-line antiretroviral regimens. Geneva: 2019.
-
- Hunt G, Steegen K, Hans L, Cassim N, MacLeod W, Carmona S. High levels of HIV drug resistance in adult patients with unsuppressed viral load, measured through routine viral load programme monitoring in South Africa; 14th INTEREST Conference. Virtual; 2020.
-
- Wijting I, Rokx C, Boucher C, van Kampen J, Pas S, de Vries-Sluijs T, et al. Dolutegravir as maintenance monotherapy for HIV (DOMONO): a phase 2, randomised non-inferiority trial. Lancet HIV. 2017;4:e547–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

