ER residential chaperone GRP78 unconventionally relocalizes to the cell surface via endosomal transport
- PMID: 33974094
- PMCID: PMC8254793
- DOI: 10.1007/s00018-021-03849-z
ER residential chaperone GRP78 unconventionally relocalizes to the cell surface via endosomal transport
Abstract
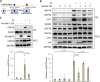
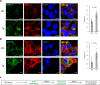
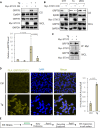
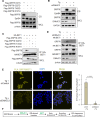
Despite new advances on the functions of ER chaperones at the cell surface, the translocation mechanisms whereby these chaperones can escape from the ER to the cell surface are just emerging. Previously we reported that in many cancer types, upon ER stress, IRE1α binds to and triggers SRC activation resulting in KDEL receptor dispersion from the Golgi and suppression of retrograde transport. In this study, using a combination of molecular, biochemical, and imaging approaches, we discovered that in colon and lung cancer, upon ER stress, ER chaperones, such as GRP78 bypass the Golgi and unconventionally traffic to the cell surface via endosomal transport mediated by Rab GTPases (Rab4, 11 and 15). Such unconventional transport is driven by membrane fusion between ER-derived vesicles and endosomes requiring the v-SNARE BET1 and t-SNARE Syntaxin 13. Furthermore, GRP78 loading into ER-derived vesicles requires the co-chaperone DNAJC3 that is regulated by ER-stress induced PERK-AKT-mTOR signaling.
Keywords: Endoplasmic reticulum stress; Endosome; GRP78; Unconventional trafficking.
Conflict of interest statement
The authors do not have any competing interests.
Figures




References
-
- Gopal U. (2018) The endoplasmic reticulum chaperone GRP78 also functions as a cell surface signaling receptor. In: Cell surface GRP78, a new paradigm in signal transduction biology, pp 9–40. 10.1016/B978-0-12-812351-5.00002-7
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

