Signaling-Biased and Constitutively Active Dopamine D2 Receptor Variant
- PMID: 33974399
- PMCID: PMC8528033
- DOI: 10.1021/acschemneuro.0c00712
Signaling-Biased and Constitutively Active Dopamine D2 Receptor Variant
Abstract
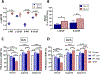
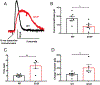
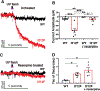
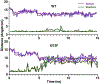
A dopamine D2 receptor mutation was recently identified in a family with a novel hyperkinetic movement disorder. Compared to the wild type D2 receptor, the novel allelic variant D2-I212F activates a Gαi1β1γ2 heterotrimer with higher potency and modestly enhanced basal activity in human embryonic kidney (HEK) 293 cells and has decreased capacity to recruit arrestin3. We now report that omitting overexpressed G protein-coupled receptor kinase-2 (GRK2) decreased the potency and efficacy of quinpirole for arrestin recruitment. The relative efficacy of quinpirole for arrestin recruitment to D2-I212F compared to D2-WT was considerably lower without overexpressed GRK2 than with added GRK2. D2-I212F exhibited higher basal activation of GαoA than Gαi1 but little or no increase in the potency of quinpirole relative to D2-WT. Other signs of D2-I212F constitutive activity for G protein-mediated signaling, in addition to basal activation of Gαi/o, were enhanced basal inhibition of forskolin-stimulated cyclic AMP accumulation that was reversed by the inverse agonists sulpiride and spiperone and a ∼4-fold increase in the apparent affinity of D2-I212F for quinpirole, determined from competition binding assays. In mouse midbrain slices, inhibition of tonic current by the inverse agonist sulpiride in dopamine neurons expressing D2-I212F was consistent with our hypothesis of enhanced constitutive activity and sensitivity to dopamine relative to D2-WT. Molecular dynamics simulations with D2 receptor models suggested that an ionic lock between the cytoplasmic ends of the third and sixth α-helices that constrains many G protein-coupled receptors in an inactive conformation spontaneously breaks in D2-I212F. Overall, these results confirm that D2-I212F is a constitutively active and signaling-biased D2 receptor mutant and also suggest that the effect of the likely pathogenic variant in a given brain region will depend on the nature of G protein and GRK expression.
Keywords: D2 receptor; Dopamine; G protein; allelic variant; arrestin; biased signaling; constitutive activity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Beaulieu JM; Sotnikova TD; Marion S; Lefkowitz RJ; Gainetdinov RR; Caron MG, An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 2005, 122 (2), 261–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

