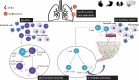
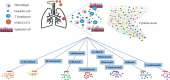
Perspectives on the use and risk of adverse events associated with cytokine-storm targeting antibodies and challenges associated with development of novel monoclonal antibodies for the treatment of COVID-19 clinical cases
- PMID: 33974497
- PMCID: PMC8127167
- DOI: 10.1080/21645515.2021.1908060
Perspectives on the use and risk of adverse events associated with cytokine-storm targeting antibodies and challenges associated with development of novel monoclonal antibodies for the treatment of COVID-19 clinical cases
Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the novel coronavirus disease 2019 (COVID-19) pandemic that lacks globally accessible effective antivirals or extensively available vaccines. Numerous clinical trials are exploring the applicability of repurposed monoclonal antibodies (mAbs) targeting cytokines that cause adverse COVID-19-related pathologies, and novel mAbs directly targeting SARS-CoV-2. However, comorbidities and the incidence of cytokine storm (CS)-associated pathological complexities in some COVID-19 patients may limit the clinical use of these drugs. Additionally, CS-targeting mAbs have the potential to cause adverse events that restrict their applicability in patients with comorbidities. Novel mAbs targeting SARS-CoV-2 require pharmacological and toxicological characterization before a marketable product becomes available. The affordability of novel mAbs across the global economic spectrum may seriously limit their accessibility. This review presents a perspective on antibody-based research efforts and their limitations for COVID-19.
Keywords: COVID-19; adverse effects; immunotherapeutics; monoclonal antibodies.
Figures
Similar articles
-
Tocilizumab in SARS-CoV-2 Patients with the Syndrome of Cytokine Storm: A Narrative Review.Rev Recent Clin Trials. 2021;16(2):138-145. doi: 10.2174/1574887115666200917110954. Rev Recent Clin Trials. 2021. PMID: 32940187 Review.
-
Monoclonal antibody therapy in COVID-19.J Biol Regul Homeost Agents. 2021 Mar-Apr;35(2):423-427. doi: 10.23812/Conti_Edit_35_2_1. J Biol Regul Homeost Agents. 2021. PMID: 33904269
-
Monoclonal Antibodies and their Target Specificity Against SARS-CoV-2 Infections: Perspectives and Challenges.Recent Pat Biotechnol. 2022;16(1):64-78. doi: 10.2174/1872208316666220106110014. Recent Pat Biotechnol. 2022. PMID: 34994337 Review.
-
Cytokines and Chemokines in SARS-CoV-2 Infections-Therapeutic Strategies Targeting Cytokine Storm.Biomolecules. 2021 Jan 12;11(1):91. doi: 10.3390/biom11010091. Biomolecules. 2021. PMID: 33445810 Free PMC article. Review.
-
Storm at the Time of Corona: A Glimpse at the Current Understanding and Therapeutic Opportunities of the SARS-CoV-2 Cytokine Storm.Curr Pharm Des. 2021;27(13):1549-1552. doi: 10.2174/1381612826666201125102649. Curr Pharm Des. 2021. PMID: 33238863
Cited by
-
Endothelial Dysfunction, Inflammation, and Oxidative Stress in COVID-19-Mechanisms and Therapeutic Targets.Oxid Med Cell Longev. 2021 Aug 21;2021:8671713. doi: 10.1155/2021/8671713. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34457119 Free PMC article. Review.
-
Review of therapeutic mechanisms and applications based on SARS-CoV-2 neutralizing antibodies.Front Microbiol. 2023 Mar 16;14:1122868. doi: 10.3389/fmicb.2023.1122868. eCollection 2023. Front Microbiol. 2023. PMID: 37007494 Free PMC article. Review.
-
Construction of a Hantaan Virus Phage Antibody Library and Screening for Potential Neutralizing Activity.Viruses. 2023 Apr 23;15(5):1034. doi: 10.3390/v15051034. Viruses. 2023. PMID: 37243121 Free PMC article.
-
Research Progress on Spike-Dependent SARS-CoV-2 Fusion Inhibitors and Small Molecules Targeting the S2 Subunit of Spike.Viruses. 2024 Apr 30;16(5):712. doi: 10.3390/v16050712. Viruses. 2024. PMID: 38793593 Free PMC article. Review.
References
-
- Johns Hopkins University and medicine . COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU); 2020.
-
- NIH-What’s New in the Guidelines . COVID-19 treatment guidelines- 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous