A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19
- PMID: 33974559
- PMCID: PMC8245169
- DOI: 10.1172/JCI150646
A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19
Abstract
BACKGROUNDAlthough convalescent plasma has been widely used to treat severe coronavirus disease 2019 (COVID-19), data from randomized controlled trials that support its efficacy are limited.METHODSWe conducted a randomized, double-blind, controlled trial among adults hospitalized with severe and critical COVID-19 at 5 sites in New York City (USA) and Rio de Janeiro (Brazil). Patients were randomized 2:1 to receive a single transfusion of either convalescent plasma or normal control plasma. The primary outcome was clinical status at 28 days following randomization, measured using an ordinal scale and analyzed using a proportional odds model in the intention-to-treat population.RESULTSOf 223 participants enrolled, 150 were randomized to receive convalescent plasma and 73 to receive normal control plasma. At 28 days, no significant improvement in the clinical scale was observed in participants randomized to convalescent plasma (OR 1.50, 95% confidence interval [CI] 0.83-2.68, P = 0.180). However, 28-day mortality was significantly lower in participants randomized to convalescent plasma versus control plasma (19/150 [12.6%] versus 18/73 [24.6%], OR 0.44, 95% CI 0.22-0.91, P = 0.034). The median titer of anti-SARS-CoV-2 neutralizing antibody in infused convalescent plasma units was 1:160 (IQR 1:80-1:320). In a subset of nasopharyngeal swab samples from Brazil that underwent genomic sequencing, no evidence of neutralization-escape mutants was detected.CONCLUSIONIn adults hospitalized with severe COVID-19, use of convalescent plasma was not associated with significant improvement in day 28 clinical status. However, convalescent plasma was associated with significantly improved survival. A possible explanation is that survivors remained hospitalized at their baseline clinical status.TRIAL REGISTRATIONClinicalTrials.gov, NCT04359810.FUNDINGAmazon Foundation, Skoll Foundation.
Keywords: COVID-19; Hypoxia.
Conflict of interest statement
Figures
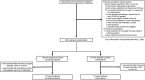

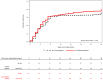

References
-
- U.S. Centers for Disease Control and Prevention. COVIDView: A Weekly Surveillance Summary of U.S. COVID-19 Activity. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html Updated May 7, 2021. Accessed May 10, 2021.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

