Prevalence and risk factors of schistosomiasis among primary school children in four selected regions of The Gambia
- PMID: 33974623
- PMCID: PMC8139473
- DOI: 10.1371/journal.pntd.0009380
Prevalence and risk factors of schistosomiasis among primary school children in four selected regions of The Gambia
Abstract
Background: The Gambia initiated a control programme for schistosomiasis in 2015. In light of this, recent and comprehensive data on schistosomiasis is required to effectively guide the control programme. This study aimed to evaluate the prevalence and associated risk factors of schistosomiasis among primary school children in The Gambia.
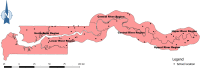
Methods: We utilised data from a previous study conducted in 2015 in 4 regions of The Gambia: North Bank Region (NBR), Lower River Region (LRR), Central River Region (CRR) and Upper River Region (URR). In the parent study, ten schools were selected randomly from each region. Urine and stool samples collected from 25 boys and 25 girls (7-14 years) in each school were examined for urinary schistosomiasis (Schistosoma haematobium infection) and intestinal schistosomiasis (Schistosoma mansoni infection) using urine filtration, dipstick and Kato-Katz methods.
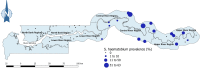
Principal findings: Urinary schistosomiasis had an overall prevalence of 10.2% while intestinal schistosomiasis had a prevalence of 0.3% among the sampled school children. Prevalence of urinary schistosomiasis was significantly different among regions (χ 2 = 279.958, df = 3, p < 0.001), with CRR (27.6%) being the most endemic region, followed by URR (12.0%), then LRR (0.6%), and NBR (0.0%). Prevalence of intestinal schistosomiasis was also significantly variable among regions, with 4 of the 5 positive cases detected in CRR and 1 case in URR. Every school sampled in CRR had at least one student infected with S. haematobium, 50% of schools in URR had S. haematobium infection, and just one school in LRR had S. haematobium infection. While S. haematobium infection was significantly higher in boys (χ 2 = 4.440, df = 1, p = 0.035), no significant difference in infection rate was observed among age groups (χ 2 = 0.882, df = 2, p = 0.643). Two of the 5 students infected with S. mansoni were boys and 3 were girls. Four of these 5 students were in the 10-12 years age group and 1 was in the 7-9 years age group. Macrohaematuria and microhaematuria were found to be statistically associated with presence of S. haematobium eggs in urine. Being a male was a risk factor of S. haematobium infection. Bathing, playing and swimming in water bodies were found to pose less risk for S. haematobium infection, indicating that the true water contact behaviour of children was possibly underrepresented.
Conclusion: The findings of this study provide invaluable information on the prevalence of schistosomiasis in The Gambia. This was useful for the schistosomiasis control efforts of the country, as it guided mass drug administration campaigns in eligible districts in the study area. More studies on S. mansoni and its intermediate snail hosts are required to establish its true status in The Gambia. As children sometimes tend to provide responses that potentially please the research or their teacher, data collection frameworks and approaches that ensure true responses in studies involving children should be devised and used.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- WHO. Working to overcome the global impact of neglected tropical diseases: First WHO report on neglected tropical diseases [Internet]. WHO. 2010. [Cited 2020 Aug 2]. Available from: 10.1177/1757913912449575 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

