Ehrlichia TRP effectors: moonlighting, mimicry and infection
- PMID: 33974702
- PMCID: PMC8112483
- DOI: 10.1093/femspd/ftab026
Ehrlichia TRP effectors: moonlighting, mimicry and infection
Abstract
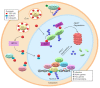
Intracellular bacteria have evolved various strategies to evade host defense mechanisms. Remarkably, the obligately intracellular bacterium, Ehrlichia chaffeensis, hijacks host cell processes of the mononuclear phagocyte to evade host defenses through mechanisms executed in part by tandem repeat protein (TRP) effectors secreted by the type 1 secretion system. In the past decade, TRP120 has emerged as a model moonlighting effector, acting as a ligand mimetic, nucleomodulin and ubiquitin ligase. These defined functions illuminate the diverse roles TRP120 plays in exploiting and manipulating host cell processes, including cytoskeletal organization, vesicle trafficking, cell signaling, transcriptional regulation, post-translational modifications, autophagy and apoptosis. This review will focus on TRP effectors and their expanding roles in infection and provide perspective on Ehrlichia chaffeensis as an invaluable model organism for understanding infection strategies of obligately intracellular bacteria.
Keywords: Ehrlichia chaffeensis; effector proteins; effector–pathogen interactions; intracellular bacteria; moonlighting; tandem repeat proteins.
© The Author(s) 2021. Published by Oxford University Press on behalf of FEMS.
Figures


References
-
- Andersson JO, Andersson SG. Genome degradation is an ongoing process in Rickettsia. Mol Biol Evol. 1999;16:1178–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

