Albumin Conjugates of Thiosemicarbazone and Imidazole-2-thione Prochelators: Iron Coordination and Antiproliferative Activity
- PMID: 33974730
- PMCID: PMC8448912
- DOI: 10.1002/cmdc.202100278
Albumin Conjugates of Thiosemicarbazone and Imidazole-2-thione Prochelators: Iron Coordination and Antiproliferative Activity
Abstract
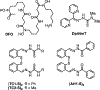
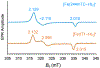
The central role of iron in tumor progression and metastasis motivates the development of iron-binding approaches in cancer chemotherapy. Disulfide-based prochelators are reductively activated upon cellular uptake to liberate thiol chelators responsible for iron sequestration. Herein, a trimethyl thiosemicarbazone moiety and the imidazole-2-thione heterocycle are incorporated in this prochelator design. Iron binding of the corresponding tridentate chelators leads to the stabilization of a low-spin ferric center in 2 : 1 ligand-to-metal complexes. Native mass spectrometry experiments show that the prochelators form stable disulfide conjugates with bovine serum albumin, thus affording novel bioconjugate prochelator systems. Antiproliferative activities at sub-micromolar levels are recorded in a panel of breast, ovarian and colorectal cancer cells, along with significantly lower activity in normal fibroblasts.
Keywords: albumin; cancer; imidazole-2-thione; iron chelation; thiosemicarbazone.
© 2021 Wiley-VCH GmbH.
Figures
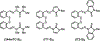
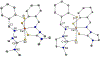

Similar articles
-
Thiol-Reactive Arylsulfonate Masks for Phenolate Donors in Antiproliferative Iron Prochelators.Inorg Chem. 2022 Dec 12;61(49):19974-19982. doi: 10.1021/acs.inorgchem.2c03250. Epub 2022 Dec 1. Inorg Chem. 2022. PMID: 36455205 Free PMC article.
-
Intracellular reduction/activation of a disulfide switch in thiosemicarbazone iron chelators.Metallomics. 2014 Oct;6(10):1905-12. doi: 10.1039/c4mt00153b. Epub 2014 Aug 7. Metallomics. 2014. PMID: 25100578 Free PMC article.
-
2-Acetylpyridine thiosemicarbazones are potent iron chelators and antiproliferative agents: redox activity, iron complexation and characterization of their antitumor activity.J Med Chem. 2009 Mar 12;52(5):1459-70. doi: 10.1021/jm801585u. J Med Chem. 2009. PMID: 19216562
-
Thiosemicarbazones as Potent Anticancer Agents and their Modes of Action.Mini Rev Med Chem. 2020;20(8):638-661. doi: 10.2174/1389557519666191029130310. Mini Rev Med Chem. 2020. PMID: 31660812 Review.
-
An insight into recent developments in imidazole based heterocyclic compounds as anticancer agents: Synthesis, SARs, and mechanism of actions.Eur J Med Chem. 2024 Dec 15;280:116896. doi: 10.1016/j.ejmech.2024.116896. Epub 2024 Sep 21. Eur J Med Chem. 2024. PMID: 39366252 Review.
Cited by
-
Aroylhydrazone Glycoconjugate Prochelators Exploit Glucose Transporter 1 (GLUT1) to Target Iron in Cancer Cells.ACS Med Chem Lett. 2022 Aug 18;13(9):1452-1458. doi: 10.1021/acsmedchemlett.2c00250. eCollection 2022 Sep 8. ACS Med Chem Lett. 2022. PMID: 36105345 Free PMC article.
-
Design of Tetrazolium Cations for the Release of Antiproliferative Formazan Chelators in Mammalian Cells.J Am Chem Soc. 2023 Jul 19;145(28):15197-15206. doi: 10.1021/jacs.3c02033. Epub 2023 Jul 6. J Am Chem Soc. 2023. PMID: 37410992 Free PMC article.
-
The Role of Iron Chelation Therapy in Colorectal Cancer: A Systematic Review on Its Mechanisms and Therapeutic Potential.Cancer Med. 2025 Jul;14(13):e71019. doi: 10.1002/cam4.71019. Cancer Med. 2025. PMID: 40607631 Free PMC article. Review.
-
Quinoline-based tetrazolium prochelators: formazan release, iron sequestration, and antiproliferative efficacy in cancer cells.Chem Commun (Camb). 2024 Jun 11;60(48):6150-6153. doi: 10.1039/d4cc01523a. Chem Commun (Camb). 2024. PMID: 38804255 Free PMC article.
-
Targeting iron to contrast cancer progression.Curr Opin Chem Biol. 2023 Jun;74:102315. doi: 10.1016/j.cbpa.2023.102315. Epub 2023 May 13. Curr Opin Chem Biol. 2023. PMID: 37187095 Free PMC article. Review.
References
-
- Bauer M, Eickhoff JC, Gould MN, Mundhenke C, Maass N, Friedl A, Breast Cancer Res. Treat 2008, 108, 389–397. - PubMed
MeSH terms
Substances
Grants and funding
- P30 ES006694/ES/NIEHS NIH HHS/United States
- P30CA023074/National Cancer Institute of the National Institutes of Health
- R35GM128624/US National Institute of General Medical Sciences of the National Institutes of Health
- R01GM127646/US National Institute of General Medical Sciences of the National Institutes of Health
- P30 CA023074/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

