Albumin Conjugates of Thiosemicarbazone and Imidazole-2-thione Prochelators: Iron Coordination and Antiproliferative Activity
- PMID: 33974730
- PMCID: PMC8448912
- DOI: 10.1002/cmdc.202100278
Albumin Conjugates of Thiosemicarbazone and Imidazole-2-thione Prochelators: Iron Coordination and Antiproliferative Activity
Abstract
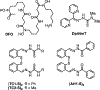
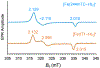
The central role of iron in tumor progression and metastasis motivates the development of iron-binding approaches in cancer chemotherapy. Disulfide-based prochelators are reductively activated upon cellular uptake to liberate thiol chelators responsible for iron sequestration. Herein, a trimethyl thiosemicarbazone moiety and the imidazole-2-thione heterocycle are incorporated in this prochelator design. Iron binding of the corresponding tridentate chelators leads to the stabilization of a low-spin ferric center in 2 : 1 ligand-to-metal complexes. Native mass spectrometry experiments show that the prochelators form stable disulfide conjugates with bovine serum albumin, thus affording novel bioconjugate prochelator systems. Antiproliferative activities at sub-micromolar levels are recorded in a panel of breast, ovarian and colorectal cancer cells, along with significantly lower activity in normal fibroblasts.
Keywords: albumin; cancer; imidazole-2-thione; iron chelation; thiosemicarbazone.
© 2021 Wiley-VCH GmbH.
Figures
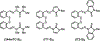
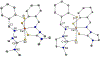

References
-
- Bauer M, Eickhoff JC, Gould MN, Mundhenke C, Maass N, Friedl A, Breast Cancer Res. Treat 2008, 108, 389–397. - PubMed
MeSH terms
Substances
Grants and funding
- P30 ES006694/ES/NIEHS NIH HHS/United States
- P30CA023074/National Cancer Institute of the National Institutes of Health
- R35GM128624/US National Institute of General Medical Sciences of the National Institutes of Health
- R01GM127646/US National Institute of General Medical Sciences of the National Institutes of Health
- P30 CA023074/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

