Plasticity of stereotyped birdsong driven by chronic manipulation of cortical-basal ganglia activity
- PMID: 33974850
- PMCID: PMC8222193
- DOI: 10.1016/j.cub.2021.04.030
Plasticity of stereotyped birdsong driven by chronic manipulation of cortical-basal ganglia activity
Abstract
Cortical-basal ganglia (CBG) circuits are critical for motor learning and performance, and are a major site of pathology. In songbirds, a CBG circuit regulates moment-by-moment variability in song and also enables song plasticity. Studies have shown that variable burst firing in LMAN, the output nucleus of this CBG circuit, actively drives acute song variability, but whether and how LMAN drives long-lasting changes in song remains unclear. Here, we ask whether chronic pharmacological augmentation of LMAN bursting is sufficient to drive plasticity in birds singing stereotyped songs. We show that altered LMAN activity drives cumulative changes in acoustic structure, timing, and sequencing over multiple days, and induces repetitions and silent pauses reminiscent of human stuttering. Changes persisted when LMAN was subsequently inactivated, indicating plasticity in song motor regions. Following cessation of pharmacological treatment, acoustic features and song sequence gradually recovered to their baseline values over a period of days to weeks. Together, our findings show that augmented bursting in CBG circuitry drives plasticity in well-learned motor skills, and may inform treatments for basal ganglia movement disorders.
Keywords: basal ganglia; motor performance; motor variability; movement disorders; sequence variability; songbird; stuttering; vocal learning; vocal plasticity; zebra finch.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



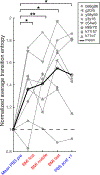


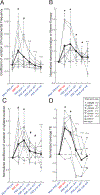
Comment in
-
Basal ganglia: Bursting with song.Curr Biol. 2021 Jun 21;31(12):R791-R793. doi: 10.1016/j.cub.2021.04.064. Curr Biol. 2021. PMID: 34157263
References
-
- Kotz SA & Schwartze M (2010). Cortical speech processing unplugged: A timely subcortico-cortical framework. Trends Cogn. Sci. 14, 392–399. - PubMed
-
- Alm PA (2004). Stuttering and the basal ganglia circuits: A critical review of possible relations. J. Commun. Disord. 37, 325–369. - PubMed
-
- Ramig LO, Fox C & Sapir S (2008). Speech treatment for Parkinson’s disease. Expert Rev. Neurother. 8, 297–309. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

