Structural insight into SARS-CoV-2 neutralizing antibodies and modulation of syncytia
- PMID: 33974910
- PMCID: PMC8064868
- DOI: 10.1016/j.cell.2021.04.033
Structural insight into SARS-CoV-2 neutralizing antibodies and modulation of syncytia
Abstract
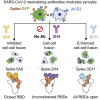
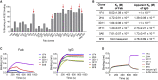
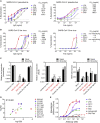
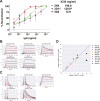
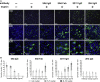
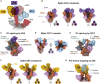
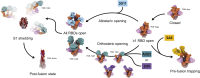
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is initiated by binding of the viral Spike protein to host receptor angiotensin-converting enzyme 2 (ACE2), followed by fusion of viral and host membranes. Although antibodies that block this interaction are in emergency use as early coronavirus disease 2019 (COVID-19) therapies, the precise determinants of neutralization potency remain unknown. We discovered a series of antibodies that potently block ACE2 binding but exhibit divergent neutralization efficacy against the live virus. Strikingly, these neutralizing antibodies can inhibit or enhance Spike-mediated membrane fusion and formation of syncytia, which are associated with chronic tissue damage in individuals with COVID-19. As revealed by cryoelectron microscopy, multiple structures of Spike-antibody complexes have distinct binding modes that not only block ACE2 binding but also alter the Spike protein conformational cycle triggered by ACE2 binding. We show that stabilization of different Spike conformations leads to modulation of Spike-mediated membrane fusion with profound implications for COVID-19 pathology and immunity.
Keywords: COVID-19; SARS-CoV; SARS-CoV-2; Spike protein; coronavirus; phage display; receptor binding domain (RBD); recombinant monoclonal antibody; syncytia.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests B.W., W.-H.L., C.-W.H., Y.H., P.M.L.N., E.Z.X.N., H.C.T., C.Y.L., R.A.M., M.K.S., F.J.T., Y.Y.C.Y., and C.-I.W. are listed as inventors of a filed patent for all 27 monoclonal antibodies mentioned in this manuscript.
Figures



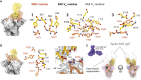

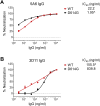
References
-
- Burton D.R., Saphire E.O., Parren P.W. A model for neutralization of viruses based on antibody coating of the virion surface. Curr. Top. Microbiol. Immunol. 2001;260:109–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

