How to interpret and use COVID-19 serology and immunology tests
- PMID: 33975005
- PMCID: PMC8106522
- DOI: 10.1016/j.cmi.2021.05.001
How to interpret and use COVID-19 serology and immunology tests
Abstract
Background: Although molecular tests are considered the reference standard for coronavirus disease 2019 (COVID-19) diagnostics, serological and immunological tests may be useful in specific settings.
Objectives: This review summarizes the underlying principles and performance of COVID-19 serological and immunological testing.
Sources: Selected peer-reviewed publications on COVID-19 related serology and immunology published between December 2019 and March 2021.
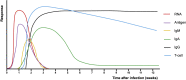
Content: Serological tests are highly specific but heterogeneous in their sensitivity for the diagnosis of COVID-19. For certain indications, including delayed disease presentations, serological tests can have added value. The presence of antibodies against SARS-CoV-2 may indicate a recent or past COVID-19 infection. Lateral flow immunoassay (LFIA) antibody tests have the advantages of being easy and fast to perform, but many have a low sensitivity in acute settings. Enzyme-linked immunosorbent assay (ELISA) and chemiluminescence immunoassays (CLIAs) have higher sensitivities. Besides humoral immunity, cellular immunity is also essential for successful host defences against viruses. Enzyme-linked immunospot (ELISpot) assays can be used to measure T-cell responses against SARS-CoV-2. The presence of cross-reactive SARS-CoV-2-specific T cells in never exposed patients suggests the possibility of cellular immunity induced by other circulating coronaviruses. T-cell responses against SARS-CoV-2 have also been detected in recovered COVID-19 patients with no detectable antibodies.
Implications: Serological and immunological tests are primarily applied for population-based seroprevalence studies to evaluate the effectiveness of COVID-19 control measures and increase our understanding of the immunology behind COVID-19. Combining molecular diagnostics with serological tests may optimize the detection of COVID-19. As not all infected patients will develop antibodies against SARS-CoV-2, assessment of cellular immunity may provide complementary information on whether a patient has been previously infected with COVID-19. More studies are needed to understand the correlations of these serological and immunological parameters with protective immunity, taking into account the different circulating virus variants.
Keywords: Antibodies; COVID-19; Immunity; SARS-CoV-2; Serology; T cell.
Copyright © 2021 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures
References
-
- Long Q.-X., Liu B.-Z., Deng H.-J., Wu G.-C., Deng K., Chen Y.-K., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

