Early antibody response in health-care professionals after two doses of SARS-CoV-2 mRNA vaccine (BNT162b2)
- PMID: 33975007
- PMCID: PMC8106520
- DOI: 10.1016/j.cmi.2021.05.004
Early antibody response in health-care professionals after two doses of SARS-CoV-2 mRNA vaccine (BNT162b2)
Abstract
Objectives: Data on the immune response after two doses of BNT162b2 are so far limited. Previously infected individuals were excluded from pivotal clinical trials and the optimum dose regimen in this population has not been clearly studied. The CRO-VAX HCP study aims to investigate the early antibody response in a population of health-care professionals having received two doses of the BNT162b2 mRNA coronavirus disease 2019 (COVID-19) vaccine.
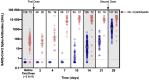
Methods: The CRO-VAX HCP study is a multicentre, prospective, interventional study conducted in several sites in Belgium. The study included 231 health-care professional volunteers who received the two-dose regimen of the BNT162b2 mRNA COVID-19 vaccine. Of these, 73 were previously infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and 158 were uninfected and seronegative. In the first group, blood samples were collected at baseline and after 2, 4, 7, 10, 14, 21 and 28 days. In the second group, samples were obtained at baseline and after 14 and 28 days. Antibodies against the SARS-CoV-2 nucleocapsid and the receptor binding domain of the S1 subunit of the spike protein were measured in all individuals at different time-points.
Results: In uninfected individuals, 95.5% (95% CI 91.0%-98.2%) developed anti-spike antibodies after 14 days and a 24.9-fold rise (95% CI 21.4%-28.9%) in antibody titre was observed after the second dose. In previously infected individuals, peak antibody response was reached after 7 days (i.e. 6347 U/mL) and the second dose did not lead to significantly higher antibody titres (i.e. 8856-11 911 U/mL). Antibody titres were higher in previously infected individuals.
Conclusions: This study supports the concept that a single dose of BNT162b2 would be sufficient in previously infected individuals.
Keywords: Antibody; BNT162b2; Coronavirus disease 2019; Humoral response; Severe acute respiratory syndrome coronavirus 2; Vaccine.
Copyright © 2021 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

