Ultrasound-assisted soil washing processes for the remediation of heavy metals contaminated soils: The mechanism of the ultrasonic desorption
- PMID: 33975185
- PMCID: PMC8122358
- DOI: 10.1016/j.ultsonch.2021.105574
Ultrasound-assisted soil washing processes for the remediation of heavy metals contaminated soils: The mechanism of the ultrasonic desorption
Abstract
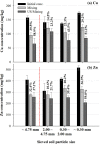
Ultrasound-assisted soil washing processes were investigated for the removal of heavy metals (Cu, Pb, and Zn) in real contaminated soils using HCl and EDTA. The ultrasound-assisted soil washing (US/Mixing) process was compared with the conventional soil washing (Mixing) process based on the mechanical mixing. High removal efficiency (44.8% for HCl and 43.2% for EDTA) for the metals was obtained for the most extreme conditions (HCl 1.0 M or EDTA 0.1 M and L:S = 10:1) in the Mixing process. With the aide of ultrasound, higher removal efficiency (57.9% for HCl and 50.0% for EDTA) was obtained in the same extreme conditions and similar or higher removal efficiency (e.g., 54.7% for HCl 0.5 M and L:S = 10:1 and 50.5% for EDTA 0.05 M and L:S = 5:1) was achieved even in less extreme conditions (lower HCl or EDTA concentration and L:S ratio). Therefore, it was revealed that the US/Mixing was advantageous over the conventional Mixing processes in terms of metal removal efficiency, consumption of chemicals, amount of generated washing leachate, and volume/size of washing reactor. In addition, the heavy metals removal was enhanced for the smaller soil particles in the US/Mixing process. It was due to more violent movement of smaller particles in slurry phase and more violent sonophysical effects. In order to understand the mechanism of ultrasonic desorption, the desorption test was conducted using the paint-coated beads with three sizes (1, 2, and 4 mm) for the free and attached conditions. It was found that no significant desorption/removal of paint from the beads was observed without the movement of beads in the water including floatation, collision, and scrubbing. Thus, it was suggested that the simultaneous application of the ultrasound and mechanical mixing could enhance the physical movement of the particles significantly and the very high removal/desorption could be attained.
Keywords: Cavitation; EDTA; HCl; Heavy metals; Soil washing; Ultrasonic desorption.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Mahamuni N.N., Adewuyi Y.G. Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: A review with emphasis on cost estimation. Ultrason. Sonochem. 2010;17(6):990–1003. - PubMed
-
- Son Y., Lim M., Khim J., Kim L.H., Ashokkumar M. Comparison of calorimetric energy and cavitation energy for the removal of bisphenol-A: The effects of frequency and liquid height. Chem. Eng. J. 2012;183:39–45.
-
- Neppolian B., Doronila A., Grieser F., Ashokkumar M. Simple and Efficient Sonochemical Method for the Oxidation of Arsenic(III) to Arsenic(V) Environ. Sci. Technol. 2009;43(17):6793–6798. - PubMed
-
- Torres R.A., Pétrier C., Combet E., Carrier M., Pulgarin C. Ultrasonic cavitation applied to the treatment of bisphenol A. Effect of sonochemical parameters and analysis of BPA by-products. Ultrason. Sonochem. 2008;15(4):605–611. - PubMed
-
- Pétrier C., Combet E., Mason T. Oxygen-induced concurrent ultrasonic degradation of volatile and non-volatile aromatic compounds. Ultrason. Sonochem. 2007;14(2):117–121. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

