An SNX10-dependent mechanism downregulates fusion between mature osteoclasts
- PMID: 33975343
- PMCID: PMC8182410
- DOI: 10.1242/jcs.254979
An SNX10-dependent mechanism downregulates fusion between mature osteoclasts
Abstract
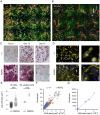
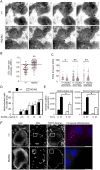
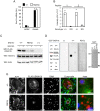
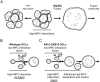
Homozygosity for the R51Q mutation in sorting nexin 10 (SNX10) inactivates osteoclasts (OCLs) and induces autosomal recessive osteopetrosis in humans and in mice. We show here that the fusion of wild-type murine monocytes to form OCLs is highly regulated, and that its extent is limited by blocking fusion between mature OCLs. In contrast, monocytes from homozygous R51Q SNX10 mice fuse uncontrollably, forming giant dysfunctional OCLs that can become 10- to 100-fold larger than their wild-type counterparts. Furthermore, mutant OCLs display reduced endocytotic activity, suggesting that their deregulated fusion is due to alterations in membrane homeostasis caused by loss of SNX10 function. This is supported by the finding that the R51Q SNX10 protein is unstable and exhibits altered lipid-binding properties, and is consistent with a key role for SNX10 in vesicular trafficking. We propose that OCL size and functionality are regulated by a cell-autonomous SNX10-dependent mechanism that downregulates fusion between mature OCLs. The R51Q mutation abolishes this regulatory activity, leading to excessive fusion, loss of bone resorption capacity and, consequently, to an osteopetrotic phenotype in vivo. This article has an associated First Person interview with the joint first authors of the paper.
Keywords: Bone; Cell fusion; Osteoclast; Osteopetrosis; SNX10.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


References
-
- Blair, H. C., Yaroslavskiy, B. B., Robinson, L. J., Mapara, M. Y., Pangrazio, A., Guo, L., Chen, K., Vezzoni, P., Tolar, J. and Orchard, P. J. (2009). Osteopetrosis with micro-lacunar resorption because of defective integrin organization. Lab. Invest. 89, 1007-1017. 10.1038/labinvest.2009.58 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

