Therapeutic administration of etoposide coincides with reduced systemic HMGB1 levels in macrophage activation syndrome
- PMID: 33975537
- PMCID: PMC8111379
- DOI: 10.1186/s10020-021-00308-0
Therapeutic administration of etoposide coincides with reduced systemic HMGB1 levels in macrophage activation syndrome
Abstract
Background: Macrophage activation syndrome (MAS) is a potentially fatal complication of systemic inflammation. HMGB1 is a nuclear protein released extracellularly during proinflammatory lytic cell death or secreted by activated macrophages, NK cells, and additional cell types during infection or sterile injury. Extracellular HMGB1 orchestrates central events in inflammation as a prototype alarmin. TLR4 and the receptor for advanced glycation end products operate as key HMGB1 receptors to mediate inflammation.
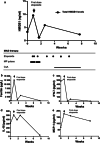
Methods: Standard ELISA and cytometric bead array-based methods were used to examine the kinetic pattern for systemic release of HMGB1, ferritin, IL-18, IFN-γ, and MCP-1 before and during treatment of four children with critical MAS. Three of the patients with severe underlying systemic rheumatic diseases were treated with biologics including tocilizumab or anakinra when MAS developed. All patients required intensive care therapy due to life-threatening illness. Add-on etoposide therapy was administered due to insufficient clinical response with standard treatment. Etoposide promotes apoptotic rather than proinflammatory lytic cell death, conceivably ameliorating subsequent systemic inflammation.
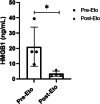
Results: This therapeutic intervention brought disease control coinciding with a decline of the increased systemic HMGB1, IFN-γ, IL-18, and ferritin levels whereas MCP-1 levels evolved independently.
Conclusion: Systemic HMGB1 levels in MAS have not been reported before. Our results suggest that the molecule is not merely a biomarker of inflammation, but most likely also contributes to the pathogenesis of MAS. These observations encourage further studies of HMGB1 antagonists. They also advocate therapeutic etoposide administration in severe MAS and provide a possible biological explanation for its mode of action.
Keywords: FHL; HLH; HMGB1; Inflammation; Macrophage activation syndrome; Pathogenesis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

