Dose adjustment of follicle-stimulating hormone (FSH) during ovarian stimulation as part of medically-assisted reproduction in clinical studies: a systematic review covering 10 years (2007-2017)
- PMID: 33975610
- PMCID: PMC8112039
- DOI: 10.1186/s12958-021-00744-x
Dose adjustment of follicle-stimulating hormone (FSH) during ovarian stimulation as part of medically-assisted reproduction in clinical studies: a systematic review covering 10 years (2007-2017)
Abstract
Background: Individualization of the follicle-stimulating hormone (FSH) starting dose is considered standard clinical practice during controlled ovarian stimulation (COS) in patients undergoing assisted reproductive technology (ART) treatment. Furthermore, the gonadotropin dose is regularly adjusted during COS to avoid hyper- or hypo-ovarian response, but limited data are currently available to characterize such adjustments. This review describes the frequency and direction (increase/decrease) of recombinant-human FSH (r-hFSH) dose adjustment reported in clinical trials.
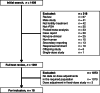
Methods: We evaluated the proportion of patients undergoing ART treatment who received ≥ 1 r-hFSH dose adjustments. The inclusion criteria included studies (published Sept 2007 to Sept 2017) in women receiving ART treatment that allowed dose adjustment within the study protocol and that reported ≥ 1 dose adjustments of r-hFSH; studies not allowing/reporting dose adjustment were excluded. Data on study design, dose adjustment and patient characteristics were extracted. Point-incidence estimates were calculated per study and overall based on pooled number of cycles with dose adjustment across studies. The Clopper-Pearson method was used to calculate 95% confidence intervals (CI) for incidence where adjustment occurred in < 10% of patients; otherwise, a normal approximation method was used.
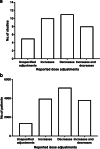
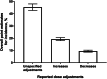
Results: Initially, 1409 publications were identified, of which 318 were excluded during initial screening and 1073 were excluded after full text review for not meeting the inclusion criteria. Eighteen studies (6630 cycles) reported dose adjustment: 5/18 studies (1359 cycles) reported data for an unspecified dose adjustment (direction not defined), in 10/18 studies (3952 cycles) dose increases were reported, and in 11/18 studies (5123 cycles) dose decreases were reported. The studies were performed in women with poor, normal and high response, with one study reporting in oocyte donors and one in obese women. The median day that dose adjustment was permitted was Day 6 after the start of treatment. The point estimates for incidence (95% CI) for unspecified dose adjustment, dose increases, and dose decreases were 45.3% (42.7, 48.0), 19.2% (18.0, 20.5), and 9.5% (8.7, 10.3), respectively.
Conclusions: This systematic review highlights that, in studies in which dose adjustment was allowed and reported, the estimated incidence of r-hFSH dose adjustments during ovarian stimulation was up to 45%.
Keywords: Assisted reproductive technology (ART) treatment; Controlled ovarian stimulation (COS); FSH dose adjustment; Follicle-stimulating hormone (FSH); Follitropin; In vitro fertilization (IVF); Medically-assisted reproduction; Recombinant-human FSH; Systematic review.
Conflict of interest statement
HR received speaker fees/honoraria/grants from MSD, Ferring, Besin, Merck KGaA (Darmstadt, Germany) and Sun Pharma; WB is an employee of Merck Serono GmbH, Darmstadt, Germany, an affiliate of Merck KGaA, Darmstadt, Germany; DD is an employee of EMD Serono Research & Development Institute, Rockland, MA, USA, a business of Merck KGaA, Darmstadt, Germany; GG reports honoraria and/or non-financial support from ObsEva, Merck KGaA (Darmstadt, Germany), MSD, Ferring, Abbott, Gedeon-Richter, Theramex, Guerbet, Finox, Glycotope and ReprodWissen GmbH; ALM received research grants and speaker fees from Merck KGaA (Darmstadt, Germany), Ferring, MSD, IBSA, Gedeon Richter, TEVA, Beckman Coulter
Figures

References
-
- Lawrenz B, Samir S, Garrido N, Melado L, Engelmann N, Fatemi HM. Luteal Coasting and Individualization of Human Chorionic Gonadotropin Dose after Gonadotropin-Releasing Hormone Agonist Triggering for Final Oocyte Maturation-A Retrospective Proof-of-Concept Study. Front Endocrinol (Lausanne) 2018;9:33. doi: 10.3389/fendo.2018.00033. - DOI - PMC - PubMed
-
- Mol BW, Bossuyt PM, Sunkara SK, Garcia Velasco JA, Venetis C, Sakkas D, Lundin K, Simón C, Taylor HS, Wan R, Longobardi S, Cottell E, D'Hooghe T. Personalized ovarian stimulation for assisted reproductive technology: study design considerations to move from hype to added value for patients. Fertil Steril. 2018;109(6):968–979. doi: 10.1016/j.fertnstert.2018.04.037. - DOI - PubMed
-
- EMA. Puregon: Sumamry of product characteristics. 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

