Phenotypic Heterogeneity and Metastasis of Breast Cancer Cells
- PMID: 33975882
- PMCID: PMC9067366
- DOI: 10.1158/0008-5472.CAN-20-1799
Phenotypic Heterogeneity and Metastasis of Breast Cancer Cells
Abstract
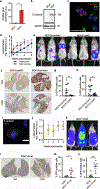
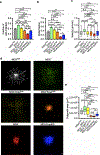
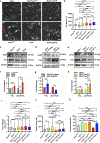
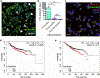
Although intratumoral genomic heterogeneity can impede cancer research and treatment, less is known about the effects of phenotypic heterogeneities. To investigate the role of cell migration heterogeneities in metastasis, we phenotypically sorted metastatic breast cancer cells into two subpopulations based on migration ability. Although migration is typically considered to be associated with metastasis, when injected orthotopically in vivo, the weakly migratory subpopulation metastasized significantly more than the highly migratory subpopulation. To investigate the mechanism behind this observation, both subpopulations were assessed at each stage of the metastatic cascade, including dissemination from the primary tumor, survival in the circulation, extravasation, and colonization. Although both subpopulations performed each step successfully, weakly migratory cells presented as circulating tumor cell (CTC) clusters in the circulation, suggesting clustering as one potential mechanism behind the increased metastasis of weakly migratory cells. RNA sequencing revealed weakly migratory subpopulations to be more epithelial and highly migratory subpopulations to be more mesenchymal. Depletion of E-cadherin expression from weakly migratory cells abrogated metastasis. Conversely, induction of E-cadherin expression in highly migratory cells increased metastasis. Clinical patient data and blood samples showed that CTC clustering and E-cadherin expression are both associated with worsened patient outcome. This study demonstrates that deconvolving phenotypic heterogeneities can reveal fundamental insights into metastatic progression. More specifically, these results indicate that migratory ability does not necessarily correlate with metastatic potential and that E-cadherin promotes metastasis in phenotypically sorted breast cancer cell subpopulations by enabling CTC clustering. SIGNIFICANCE: This study employs phenotypic cell sorting for migration to reveal a weakly migratory, highly metastatic breast cancer cell subpopulation regulated by E-cadherin, highlighting the dichotomy between cancer cell migration and metastasis.
©2021 American Association for Cancer Research.
Conflict of interest statement
Figures



References
-
- Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol. 2015;12:381–94. - PubMed
-
- Yang F, Wang Y, Li Q, Cao L, Sun Z, Jin J, et al. Intratumor heterogeneity predicts metastasis of triplenegative breast cancer. Carcinogenesis. 2017;38:900–9. - PubMed
-
- Turajlic S, Swanton C. Metastasis as an evolutionary process. Science. 2016;352:169–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

