Tribbles Homolog 3 Mediates the Development and Progression of Diabetic Retinopathy
- PMID: 33975909
- PMCID: PMC8385618
- DOI: 10.2337/db20-1268
Tribbles Homolog 3 Mediates the Development and Progression of Diabetic Retinopathy
Abstract
The current understanding of the molecular pathogenesis of diabetic retinopathy does not provide a mechanistic link between early molecular changes and the subsequent progression of the disease. In this study, we found that human diabetic retinas overexpressed TRIB3 and investigated the role of TRIB3 in diabetic retinal pathobiology in mice. We discovered that TRIB3 controlled major molecular events in early diabetic retinas via HIF1α-mediated regulation of retinal glucose flux, reprogramming cellular metabolism, and governing of inflammatory gene expression. These early molecular events further defined the development of neurovascular deficit observed in mice with diabetic retinopathy. TRIB3 ablation in the streptozotocin-induced mouse model led to significant retinal ganglion cell survival and functional restoration accompanied by a dramatic reduction in pericyte loss and acellular capillary formation. Under hypoxic conditions, TRIB3 contributed to advanced proliferative stages by significant upregulation of GFAP and VEGF expression, thus controlling gliosis and aberrant vascularization in oxygen-induced retinopathy mouse retinas. Overall, our data reveal that TRIB3 is a master regulator of diabetic retinal pathophysiology that may accelerate the onset and progression of diabetic retinopathy to proliferative stages in humans and present TRIB3 as a potentially novel therapeutic target for diabetic retinopathy.
© 2021 by the American Diabetes Association.
Figures
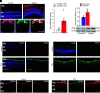

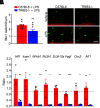

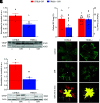
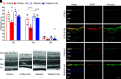

References
-
- Stitt AW, Curtis TM, Chen M, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res 2016;51:156–186 - PubMed
-
- Ord T, Ord T. Mammalian pseudokinase TRIB3 in normal physiology and disease: charting the progress in old and new avenues. Curr Protein Pept Sci 2017;18:819–842 - PubMed
-
- Fang N, Zhang W, Xu S, et al. TRIB3 alters endoplasmic reticulum stress-induced β-cell apoptosis via the NF-κB pathway. Metabolism 2014;63:822–830 - PubMed
-
- Cheng W, Mi L, Tang J, Yu W. Expression of TRB3 promotes epithelial-mesenchymal transition of MLE-12 murine alveolar type II epithelial cells through the TGF-β1/Smad3 signaling pathway. Mol Med Rep 2019;19:2869–2875 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

