Knockout RAGE alleviates cardiac fibrosis through repressing endothelial-to-mesenchymal transition (EndMT) mediated by autophagy
- PMID: 33976108
- PMCID: PMC8113558
- DOI: 10.1038/s41419-021-03750-4
Knockout RAGE alleviates cardiac fibrosis through repressing endothelial-to-mesenchymal transition (EndMT) mediated by autophagy
Abstract
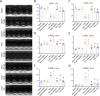
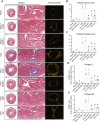
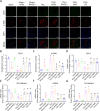
Endothelial-to-mesenchymal transition (EndMT) has been shown to contribute to cardiac fibrosis and heart failure (HF). Recent studies have demonstrated that EndMT is regulated by autophagy, and we previously showed suppression of excessive autophagy and alleviation of cardiac fibrosis in HF mice with inactivated receptor for advanced glycation end products (RAGE). Thus, we investigated whether reduced cardiac fibrosis due to RAGE knockout occurred by inhibiting EndMT mediated by excessive autophagy. We found a decrease in endothelial cells (CD31+/VE-Cadherin+) and an increase in cells co-expressing CD31 and α-smooth muscle actin (α-SMA, myofibroblast marker) at 8 weeks in heart tissue of mice subjected to transverse aortic constriction (TAC), which implied EndMT. Knockout RAGE decreased EndMT accompanied by decreased expression of autophagy-related proteins (LC3BII/I and Beclin 1), and alleviated cardiac fibrosis and improved cardiac function in TAC mice. Moreover, 3-methyladenine (3-MA) and chloroquine (CQ), inhibitors of autophagy, attenuated EndMT, and cardiac fibrosis in TAC mice. Importantly, EndMT induced by AGEs could be blocked by autophagy inhibitor in vivo and in vitro. These results suggested that AGEs/RAGE-autophagy-EndMT axis involved in the development of cardiac fibrosis and knockout RAGE ameliorated cardiac fibrosis through decreasing EndMT regulated by autophagy, which could be a promising therapeutic strategy for HF.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- MacLean J, Pasumarthi KB. Signaling mechanisms regulating fibroblast activation, phenoconversion and fibrosis in the heart. Indian J. Biochem. Biophys. 2014;51:476–482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

