Critical role of interferons in gastrointestinal injury repair
- PMID: 33976143
- PMCID: PMC8113246
- DOI: 10.1038/s41467-021-22928-0
Critical role of interferons in gastrointestinal injury repair
Abstract
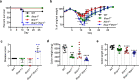
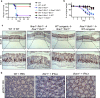
The etiology of ulcerative colitis is poorly understood and is likely to involve perturbation of the complex interactions between the mucosal immune system and the commensal bacteria of the gut, with cytokines acting as important cross-regulators. Here we use IFN receptor-deficient mice in a dextran sulfate sodium (DSS) model of acute intestinal injury to study the contributions of type I and III interferons (IFN) to the initiation, progression and resolution of acute colitis. We find that mice lacking both types of IFN receptors exhibit enhanced barrier destruction, extensive loss of goblet cells and diminished proliferation of epithelial cells in the colon following DSS-induced damage. Impaired mucosal healing in double IFN receptor-deficient mice is driven by decreased amphiregulin expression, which IFN signaling can up-regulate in either the epithelial or hematopoietic compartment. Together, these data underscore the pleiotropic functions of IFNs and demonstrate that these critical antiviral cytokines also support epithelial regeneration following acute colonic injury.
Conflict of interest statement
S.V.K. is an inventor on patents and patent applications related to IFN-λs, which have been licensed for commercial development. Other authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

