Immune evolution from preneoplasia to invasive lung adenocarcinomas and underlying molecular features
- PMID: 33976164
- PMCID: PMC8113327
- DOI: 10.1038/s41467-021-22890-x
Immune evolution from preneoplasia to invasive lung adenocarcinomas and underlying molecular features
Abstract
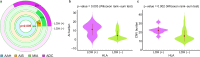
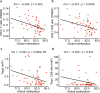
The mechanism by which anti-cancer immunity shapes early carcinogenesis of lung adenocarcinoma (ADC) is unknown. In this study, we characterize the immune contexture of invasive lung ADC and its precursors by transcriptomic immune profiling, T cell receptor (TCR) sequencing and multiplex immunofluorescence (mIF). Our results demonstrate that anti-tumor immunity evolved as a continuum from lung preneoplasia, to preinvasive ADC, minimally-invasive ADC and frankly invasive lung ADC with a gradually less effective and more intensively regulated immune response including down-regulation of immune-activation pathways, up-regulation of immunosuppressive pathways, lower infiltration of cytotoxic T cells (CTLs) and anti-tumor helper T cells (Th), higher infiltration of regulatory T cells (Tregs), decreased T cell clonality, and lower frequencies of top T cell clones in later-stages. Driver mutations, chromosomal copy number aberrations (CNAs) and aberrant DNA methylation may collectively impinge host immune responses and facilitate immune evasion, promoting the outgrowth of fit subclones in preneoplasia into dominant clones in invasive ADC.
Conflict of interest statement
J.J.Z. reports research funding from Merck, Johnson and Johnson, and consultant fees from BMS, Johnson and Johnson, AstraZeneca, Geneplus, OrigMed, Innovent outside the submitted work. I.I.W reports Honoraria from Genentech/Roche, Bayer, Bristol-Myers Squibb, Astra Zeneca/Medimmune, Pfizer, HTG Molecular, Asuragen, Merck, GlaxoSmithKline, Guardant Health, Oncocyte, Flame, and MSD; Research support from Genentech, Oncoplex, HTG Molecular, DepArray, Merck, Bristol-Myers Squibb, Medimmune, Adaptive, Adaptimmune, EMD Serono, Pfizer, Takeda, Amgen, Karus, Johnson & Johnson, Bayer, Iovance, 4D, Novartis, and Akoya. H.K. reports funding to MD Anderson Cancer Center from Johnson and Johnson. J.V.H. reports honorariums from AstraZeneca, Boehringer-Ingelheim, Catalyst, Genentech, GlaxoSmithKline, Guardant Health, Foundation medicine, Hengrui Therapeutics, Eli Lilly, Novartis, Spectrum, EMD Serono, Sanofi, Takeda, Mirati Therapeutics, BMS, BrightPath Biotherapeutics, Janssen Global Services, Nexus Health Systems, EMD Serono, Pneuma Respiratory, Kairos Venture Investments, Roche and Leads Biolabs. The other authors declare no competing interests.
Figures




Similar articles
-
Integrated whole-exome and bulk transcriptome sequencing delineates the dynamic evolution from preneoplasia to invasive lung adenocarcinoma featured with ground-glass nodules.Cancer Med. 2024 Jun;13(11):e7383. doi: 10.1002/cam4.7383. Cancer Med. 2024. PMID: 38864483 Free PMC article.
-
Genomic Landscape and Immune Microenvironment Features of Preinvasive and Early Invasive Lung Adenocarcinoma.J Thorac Oncol. 2019 Nov;14(11):1912-1923. doi: 10.1016/j.jtho.2019.07.031. Epub 2019 Aug 22. J Thorac Oncol. 2019. PMID: 31446140 Free PMC article.
-
Evolution of DNA methylome from precancerous lesions to invasive lung adenocarcinomas.Nat Commun. 2021 Jan 29;12(1):687. doi: 10.1038/s41467-021-20907-z. Nat Commun. 2021. PMID: 33514726 Free PMC article.
-
The Dark Side of IFN-γ: Its Role in Promoting Cancer Immunoevasion.Int J Mol Sci. 2017 Dec 28;19(1):89. doi: 10.3390/ijms19010089. Int J Mol Sci. 2017. PMID: 29283429 Free PMC article. Review.
-
Targeting Metabolic Bottlenecks in Lung Cancer.Trends Cancer. 2019 Aug;5(8):457-459. doi: 10.1016/j.trecan.2019.06.001. Epub 2019 Jun 27. Trends Cancer. 2019. PMID: 31421901 Review.
Cited by
-
Signature stemmed from two transcription factor families determines histological fate and regulates immune infiltration in patients with lung cancer.J Thorac Dis. 2024 Sep 30;16(9):5663-5674. doi: 10.21037/jtd-24-733. Epub 2024 Sep 13. J Thorac Dis. 2024. PMID: 39444905 Free PMC article.
-
Immunotherapy response and microenvironment provide biomarkers of immunotherapy options for patients with lung adenocarcinoma.Front Genet. 2022 Oct 25;13:1047435. doi: 10.3389/fgene.2022.1047435. eCollection 2022. Front Genet. 2022. PMID: 36386793 Free PMC article.
-
Increased Tumor Intrinsic Growth Potential and Decreased Immune Function Orchestrate the Progression of Lung Adenocarcinoma.Front Immunol. 2022 Jul 1;13:921761. doi: 10.3389/fimmu.2022.921761. eCollection 2022. Front Immunol. 2022. PMID: 35844495 Free PMC article.
-
Advances in Mapping Tumor Progression from Precancer Atlases.Cancer Prev Res (Phila). 2023 Aug 1;16(8):439-447. doi: 10.1158/1940-6207.CAPR-22-0473. Cancer Prev Res (Phila). 2023. PMID: 37167978 Free PMC article.
-
TIME for Bugs: The Immune Microenvironment and Microbes in Precancer.Cancer Prev Res (Phila). 2023 Sep 1;16(9):497-505. doi: 10.1158/1940-6207.CAPR-23-0087. Cancer Prev Res (Phila). 2023. PMID: 37428011 Free PMC article. Review.
References
-
- Aoyagi Y, et al. Accumulation of losses of heterozygosity and multistep carcinogenesis in pulmonary adenocarcinoma. Cancer Res. 2001;61:7950–7954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

