Posterior subthalamic nucleus (PSTh) mediates innate fear-associated hypothermia in mice
- PMID: 33976193
- PMCID: PMC8113537
- DOI: 10.1038/s41467-021-22914-6
Posterior subthalamic nucleus (PSTh) mediates innate fear-associated hypothermia in mice
Erratum in
-
Author Correction: Posterior subthalamic nucleus (PSTh) mediates innate fear-associated hypothermia in mice.Nat Commun. 2021 Aug 23;12(1):5153. doi: 10.1038/s41467-021-25434-5. Nat Commun. 2021. PMID: 34426579 Free PMC article. No abstract available.
Abstract
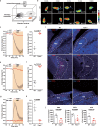
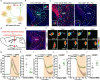
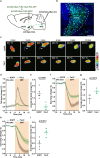
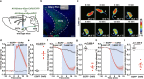
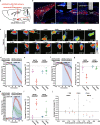
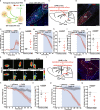
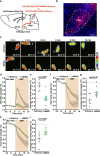
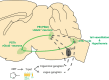
The neural mechanisms of fear-associated thermoregulation remain unclear. Innate fear odor 2-methyl-2-thiazoline (2MT) elicits rapid hypothermia and elevated tail temperature, indicative of vasodilation-induced heat dissipation, in wild-type mice, but not in mice lacking Trpa1-the chemosensor for 2MT. Here we report that Trpa1-/- mice show diminished 2MT-evoked c-fos expression in the posterior subthalamic nucleus (PSTh), external lateral parabrachial subnucleus (PBel) and nucleus of the solitary tract (NTS). Whereas tetanus toxin light chain-mediated inactivation of NTS-projecting PSTh neurons suppress, optogenetic activation of direct PSTh-rostral NTS pathway induces hypothermia and tail vasodilation. Furthermore, selective opto-stimulation of 2MT-activated, PSTh-projecting PBel neurons by capturing activated neuronal ensembles (CANE) causes hypothermia. Conversely, chemogenetic suppression of vGlut2+ neurons in PBel or PSTh, or PSTh-projecting PBel neurons attenuates 2MT-evoked hypothermia and tail vasodilation. These studies identify PSTh as a major thermoregulatory hub that connects PBel to NTS to mediate 2MT-evoked innate fear-associated hypothermia and tail vasodilation.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Morrison, S. F. & Nakamura, K. Central mechanisms for thermoregulation. Annn. Rev. Physiol.81, 285–308 (2019). - PubMed
-
- Huey RB. Temoerature, physiology, and the ecology of reptile. Deep Sea Res. I Oceanogr. Res. Pap. 2008;92:127–140.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

