A SARS-CoV-2 neutralizing antibody with extensive Spike binding coverage and modified for optimal therapeutic outcomes
- PMID: 33976198
- PMCID: PMC8113581
- DOI: 10.1038/s41467-021-22926-2
A SARS-CoV-2 neutralizing antibody with extensive Spike binding coverage and modified for optimal therapeutic outcomes
Abstract
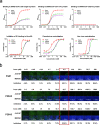
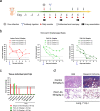
COVID-19 pandemic caused by SARS-CoV-2 constitutes a global public health crisis with enormous economic consequences. Monoclonal antibodies against SARS-CoV-2 can provide an important treatment option to fight COVID-19, especially for the most vulnerable populations. In this work, potent antibodies binding to SARS-CoV-2 Spike protein were identified from COVID-19 convalescent patients. Among them, P4A1 interacts directly with and covers majority of the Receptor Binding Motif of the Spike Receptor-Binding Domain, shown by high-resolution complex structure analysis. We further demonstrate the binding and neutralizing activities of P4A1 against wild type and mutant Spike proteins or pseudoviruses. P4A1 was subsequently engineered to reduce the potential risk for Antibody-Dependent Enhancement of infection and to extend its half-life. The engineered antibody exhibits an optimized pharmacokinetic and safety profile, and it results in complete viral clearance in a rhesus monkey model of COVID-19 following a single injection. These data suggest its potential against SARS-CoV-2 related diseases.
Conflict of interest statement
L. S., B.-Q. S., H. Z., M.-J. C., Y.-Y. L., L. S. H., and K. S. are the inventors of the pending patent application filed on the reported antibodies. L. S., B.-Q. S., H. Z., M.-J. C., J. W., M.-F. F., C.-J. P., M.-M. L., Y.-Y. L., S.-S. W., Q. Z., and F. A. are employees of HiFiBio (Hong Kong) Ltd. Y.-R. L. is an employee of Wuxi Biologics Ltd. Other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

