Community composition of microbial microcosms follows simple assembly rules at evolutionary timescales
- PMID: 33976223
- PMCID: PMC8113234
- DOI: 10.1038/s41467-021-23247-0
Community composition of microbial microcosms follows simple assembly rules at evolutionary timescales
Abstract
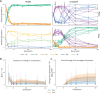
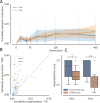
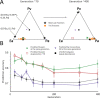
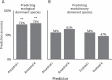
Managing and engineering microbial communities relies on the ability to predict their composition. While progress has been made on predicting compositions on short, ecological timescales, there is still little work aimed at predicting compositions on evolutionary timescales. Therefore, it is still unknown for how long communities typically remain stable after reaching ecological equilibrium, and how repeatable and predictable are changes when they occur. Here, we address this knowledge gap by tracking the composition of 87 two- and three-species bacterial communities, with 3-18 replicates each, for ~400 generations. We find that community composition typically changed during evolution, but that the composition of replicate communities remained similar. Furthermore, these changes were predictable in a bottom-up approach-changes in the composition of trios were consistent with those that occurred in pairs during coevolution. Our results demonstrate that simple assembly rules can hold even on evolutionary timescales, suggesting it may be possible to forecast the evolution of microbial communities.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Disentangling the Relative Roles of Vertical Transmission, Subsequent Colonizations, and Diet on Cockroach Microbiome Assembly.mSphere. 2021 Jan 6;6(1):e01023-20. doi: 10.1128/mSphere.01023-20. mSphere. 2021. PMID: 33408228 Free PMC article.
-
Tracking Replicate Divergence in Microbial Community Composition and Function in Experimental Microcosms.Microb Ecol. 2019 Nov;78(4):1035-1039. doi: 10.1007/s00248-019-01368-w. Epub 2019 Apr 2. Microb Ecol. 2019. PMID: 30941446
-
Impact of timing on the invasion of synthetic bacterial communities.ISME J. 2024 Jan 8;18(1):wrae220. doi: 10.1093/ismejo/wrae220. ISME J. 2024. PMID: 39498487 Free PMC article.
-
Priority effects in microbiome assembly.Nat Rev Microbiol. 2022 Feb;20(2):109-121. doi: 10.1038/s41579-021-00604-w. Epub 2021 Aug 27. Nat Rev Microbiol. 2022. PMID: 34453137 Review.
-
HOW DO MICROBIAL POPULATIONS AND COMMUNITIES FUNCTION AS MODEL SYSTEMS?Q Rev Biol. 2015 Sep;90(3):269-93. doi: 10.1086/682588. Q Rev Biol. 2015. PMID: 26591851 Review.
Cited by
-
Learning beyond-pairwise interactions enables the bottom-up prediction of microbial community structure.Proc Natl Acad Sci U S A. 2024 Feb 13;121(7):e2312396121. doi: 10.1073/pnas.2312396121. Epub 2024 Feb 5. Proc Natl Acad Sci U S A. 2024. PMID: 38315845 Free PMC article.
-
Interactions between metabolism and growth can determine the co-existence of Staphylococcus aureus and Pseudomonas aeruginosa.Elife. 2023 Apr 20;12:e83664. doi: 10.7554/eLife.83664. Elife. 2023. PMID: 37078696 Free PMC article.
-
Guided by the principles of microbiome engineering: Accomplishments and perspectives for environmental use.mLife. 2022 Nov 3;1(4):382-398. doi: 10.1002/mlf2.12043. eCollection 2022 Dec. mLife. 2022. PMID: 38818482 Free PMC article. Review.
-
Co-Existence Slows Diversification in Experimental Populations of E. coli and P. fluorescens.Environ Microbiol. 2025 Feb;27(2):e70061. doi: 10.1111/1462-2920.70061. Environ Microbiol. 2025. PMID: 39988434 Free PMC article.
-
Higher-order interactions and emergent properties of microbial communities: The power of synthetic ecology.Heliyon. 2024 Jul 9;10(14):e33896. doi: 10.1016/j.heliyon.2024.e33896. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39130413 Free PMC article. Review.
References
-
- Falkowski PG, Fenchel T, Delong EF. The microbial engines that drive earth’s biogeochemical cycles. Science. 2008;320:1034–1039. - PubMed
-
- Maukonen J, Saarela M. Microbial communities in industrial environment. Curr. Opin. Microbiol. 2009;12:238–243. - PubMed
-
- Sunagawa, S. et al. Structure and function of the global ocean microbiome. Science. 348, 1261359 (2015). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

