Molecular evolution and the decline of purifying selection with age
- PMID: 33976227
- PMCID: PMC8113359
- DOI: 10.1038/s41467-021-22981-9
Molecular evolution and the decline of purifying selection with age
Abstract
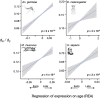
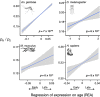
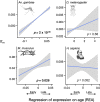
Life history theory predicts that the intensity of selection declines with age, and this trend should impact how genes expressed at different ages evolve. Here we find consistent relationships between a gene's age of expression and patterns of molecular evolution in two mammals (the human Homo sapiens and the mouse Mus musculus) and two insects (the malaria mosquito Anopheles gambiae and the fruit fly Drosophila melanogaster). When expressed later in life, genes fix nonsynonymous mutations more frequently, are more polymorphic for nonsynonymous mutations, and have shorter evolutionary lifespans, relative to those expressed early. The latter pattern is explained by a simple evolutionary model. Further, early-expressed genes tend to be enriched in similar gene ontology terms across species, while late-expressed genes show no such consistency. In humans, late-expressed genes are more likely to be linked to cancer and to segregate for dominant disease-causing mutations. Last, the effective strength of selection (Ne s) decreases and the fraction of beneficial mutations increases with a gene's age of expression. These results are consistent with the diminishing efficacy of purifying selection with age, as proposed by Medawar's classic hypothesis for the evolution of senescence, and provide links between life history theory and molecular evolution.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Age-specific patterns of genetic variance in Drosophila melanogaster. I. Mortality.Genetics. 1996 Jun;143(2):839-48. doi: 10.1093/genetics/143.2.839. Genetics. 1996. PMID: 8725232 Free PMC article.
-
Molecular footprint of Medawar's mutation accumulation process in mammalian aging.Aging Cell. 2019 Aug;18(4):e12965. doi: 10.1111/acel.12965. Epub 2019 May 6. Aging Cell. 2019. PMID: 31062469 Free PMC article.
-
"Plus-C" odorant-binding protein genes in two Drosophila species and the malaria mosquito Anopheles gambiae.Gene. 2004 Feb 18;327(1):117-29. doi: 10.1016/j.gene.2003.11.007. Gene. 2004. PMID: 14960367
-
Selection for maximum longevity in mice.Exp Gerontol. 1997 Jan-Apr;32(1-2):65-78. doi: 10.1016/s0531-5565(96)00034-4. Exp Gerontol. 1997. PMID: 9088903 Review.
-
How the analysis of genetic mutations can help us to solve basic problems in gerontology? II. Life extending genetic modifications in budding yeast S. cereviseae, fruit fly D. melanogaster and laboratory mice M. musculus.Adv Gerontol. 2003;12:46-56. Adv Gerontol. 2003. PMID: 14743601 Review.
Cited by
-
Evolution and function of developmentally dynamic pseudogenes in mammals.Genome Biol. 2022 Nov 8;23(1):235. doi: 10.1186/s13059-022-02802-y. Genome Biol. 2022. PMID: 36348461 Free PMC article.
-
VectorBase.org updates: bioinformatic resources for invertebrate vectors of human pathogens and related organisms.Curr Opin Insect Sci. 2022 Apr;50:100860. doi: 10.1016/j.cois.2021.11.008. Epub 2021 Dec 3. Curr Opin Insect Sci. 2022. PMID: 34864248 Free PMC article. Review.
-
Transcriptional and mutational signatures of the Drosophila ageing germline.Nat Ecol Evol. 2023 Mar;7(3):440-449. doi: 10.1038/s41559-022-01958-x. Epub 2023 Jan 12. Nat Ecol Evol. 2023. PMID: 36635344 Free PMC article.
-
Putative protective genomic variation in the Lithuanian population.Genet Mol Biol. 2024 Apr 15;47(2):e20230030. doi: 10.1590/1678-4685-GMB-2023-0030. eCollection 2024. Genet Mol Biol. 2024. PMID: 38626572 Free PMC article.
-
Ageing leads to reduced specificity of antimicrobial peptide responses in Drosophila melanogaster.Proc Biol Sci. 2022 Nov 30;289(1987):20221642. doi: 10.1098/rspb.2022.1642. Epub 2022 Nov 16. Proc Biol Sci. 2022. PMID: 36382522 Free PMC article.
References
-
- Medawar PB. Old age and natural death. Mod. Quart. 1946;2:30–49.
-
- Medawar, P. B. An Unsolved Problem of Biology (H.K. Lewis, 1952).
-
- Charlesworth, B. Evolution in Age-Structured Populations, 2nd edn. (Cambridge University Press, 1980).
-
- Rodriguez, J. A. et al. Antagonistic pleiotropy and mutation accumulation influence human senescence and disease. Nat. Ecol. Evol.1, 1–5 (2017). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

