Hypoperfusion of the infrapatellar fat pad and its relationship to MRI T2* relaxation time changes in a 5/6 nephrectomy model
- PMID: 33976243
- PMCID: PMC8113578
- DOI: 10.1038/s41598-021-89336-8
Hypoperfusion of the infrapatellar fat pad and its relationship to MRI T2* relaxation time changes in a 5/6 nephrectomy model
Abstract
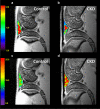
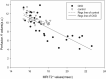
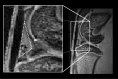
The purpose of present study was to longitudinally investigate the alterations in infrapatellar fat pad (IPFP) vascularity in 5/6 nephrectomized rats by using dynamic contrast enhanced (DCE) MRI and IPFP degeneration by using MRI T2* relaxation time. Twelve male Sprague-Dawley rats were assigned to a control group and a 5/6 nephrectomy CKD group. The right knees of all rats were longitudinally scanned by 4.7 T MRI, and serial changes in the IPFP were assessed at 0, 8, 16, 30, and 44 weeks by DCE-MRI (parameters A, kel and kep) and MRI T2* mapping. After MRI measurements, knee specimens were obtained and evaluated histologically. The CKD group had IPFPs with lower blood volume A and lower permeability kep values from 16 weeks (p < 0.05), lower venous washout kel value from 30 weeks (p < 0.001), and significantly higher T2* values reflecting adipocyte degeneration beginning at 16 weeks (p < 0.05). The histopathological results confirmed the MRI findings. Hypoperfusion and adipocytes degeneration related to CKD were demonstrated in a rodent 5/6 nephrectomy model. DCE parameters and MRI T2* can serve as imaging biomarkers of fat pad degeneration during CKD progression.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

