A novel thermostable beetle luciferase based cytotoxicity assay
- PMID: 33976304
- PMCID: PMC8113442
- DOI: 10.1038/s41598-021-89404-z
A novel thermostable beetle luciferase based cytotoxicity assay
Abstract
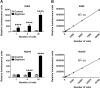
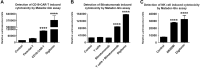
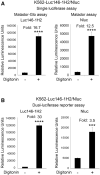
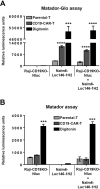
Cytotoxicity assays are essential for the testing and development of novel immunotherapies for the treatment of cancer. We recently described a novel cytotoxicity assay, termed the Matador assay, which was based on marine luciferases and their engineered derivatives. In this study, we describe the development of a new cytotoxicity assay termed 'Matador-Glo assay' which takes advantage of a thermostable variant of Click Beetle Luciferase (Luc146-1H2). Matador-Glo assay utilizes Luc146-1H2 and D-luciferin as the luciferase-substrate pair for luminescence detection. The assay involves ectopic over-expression of Luc146-1H2 in the cytosol of target cells of interest. Upon damage to the membrane integrity, the Luc146-1H2 is either released from the dead and dying cells or its activity is preferentially measured in dead and dying cells. We demonstrate that this assay is simple, fast, specific, sensitive, cost-efficient, and not labor-intensive. We further demonstrate that the Matador-Glo assay can be combined with the marine luciferase-based Matador assay to develop a dual luciferase assay for cell death detection. Finally, we demonstrate that the Luc146-1H2 expressing target cells can also be used for in vivo bioluminescence imaging applications.
Conflict of interest statement
P.M.C. is an inventor on a patent application related to the technology described in this manuscript which has been licensed to Angeles Therapeutics. P.M.C. serves as a consultant for Athelas, Oncotartis and Pancella, and is the founder of Angeles Therapeutics. All the other authors declare no competing financial interests.
Figures



Similar articles
-
Development and characterization of a novel luciferase based cytotoxicity assay.Sci Rep. 2018 Jan 9;8(1):199. doi: 10.1038/s41598-017-18606-1. Sci Rep. 2018. PMID: 29317736 Free PMC article.
-
Evaluating Brightness and Spectral Properties of Click Beetle and Firefly Luciferases Using Luciferin Analogues: Identification of Preferred Pairings of Luciferase and Substrate for In Vivo Bioluminescence Imaging.Mol Imaging Biol. 2020 Dec;22(6):1523-1531. doi: 10.1007/s11307-020-01523-7. Epub 2020 Sep 14. Mol Imaging Biol. 2020. PMID: 32926287 Free PMC article.
-
Click beetle luciferase mutant and near infrared naphthyl-luciferins for improved bioluminescence imaging.Nat Commun. 2018 Jan 9;9(1):132. doi: 10.1038/s41467-017-02542-9. Nat Commun. 2018. PMID: 29317625 Free PMC article.
-
Evolution of beetle bioluminescence: the origin of beetle luciferin.Luminescence. 2004 Jan-Feb;19(1):8-20. doi: 10.1002/bio.749. Luminescence. 2004. PMID: 14981641 Review.
-
Beyond D-luciferin: expanding the scope of bioluminescence imaging in vivo.Curr Opin Chem Biol. 2014 Aug;21:112-20. doi: 10.1016/j.cbpa.2014.07.003. Epub 2014 Aug 1. Curr Opin Chem Biol. 2014. PMID: 25078002 Free PMC article. Review.
Cited by
-
Regulatory effects of miR-28 on osteogenic differentiation of human bone marrow mesenchymal stem cells.Bioengineered. 2022 Jan;13(1):684-696. doi: 10.1080/21655979.2021.2012618. Bioengineered. 2022. PMID: 34978269 Free PMC article.
References
-
- Olsson T, Gulliksson H, Palmeborn M, Bergstrom K, Thore A. Leakage of adenylate kinase from stored blood cells. J. Appl. Biochem. 1983;5:437–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

