Engineered MATE multidrug transporters reveal two functionally distinct ion-coupling pathways in NorM from Vibrio cholerae
- PMID: 33976372
- PMCID: PMC8113278
- DOI: 10.1038/s42003-021-02081-6
Engineered MATE multidrug transporters reveal two functionally distinct ion-coupling pathways in NorM from Vibrio cholerae
Abstract
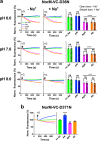
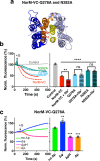
Multidrug and toxic compound extrusion (MATE) transport proteins confer multidrug resistance on pathogenic microorganisms and affect pharmacokinetics in mammals. Our understanding of how MATE transporters work, has mostly relied on protein structures and MD simulations. However, the energetics of drug transport has not been studied in detail. Many MATE transporters utilise the electrochemical H+ or Na+ gradient to drive substrate efflux, but NorM-VC from Vibrio cholerae can utilise both forms of metabolic energy. To dissect the localisation and organisation of H+ and Na+ translocation pathways in NorM-VC we engineered chimaeric proteins in which the N-lobe of H+-coupled NorM-PS from Pseudomonas stutzeri is fused to the C-lobe of NorM-VC, and vice versa. Our findings in drug binding and transport experiments with chimaeric, mutant and wildtype transporters highlight the versatile nature of energy coupling in NorM-VC, which enables adaptation to fluctuating salinity levels in the natural habitat of V. cholerae.
Conflict of interest statement
The authors declare no competing interests.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

