Depletion of kinesin motor KIF20A to target cell fate control suppresses medulloblastoma tumour growth
- PMID: 33976373
- PMCID: PMC8113472
- DOI: 10.1038/s42003-021-02075-4
Depletion of kinesin motor KIF20A to target cell fate control suppresses medulloblastoma tumour growth
Abstract
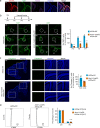
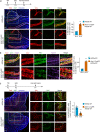
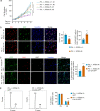
During mammalian brain development, neural progenitor cells proliferate extensively but can ensure the production of correct numbers of various types of mature cells by balancing symmetric proliferative versus asymmetric differentiative cell divisions. This process of cell fate determination may be harnessed for developing cancer therapy. Here, we test this idea by targeting KIF20A, a mitotic kinesin crucial for the control of cell division modes, in a genetic model of medulloblastoma (MB) and human MB cells. Inducible Kif20a knockout in both normal and MB-initiating granule neuron progenitors (GNPs) causes early cell cycle exit and precocious neuronal differentiation without causing cytokinesis failure and suppresses the development of Sonic Hedgehog (SHH)-activated MB. Inducible KIF20A knockdown in human MB cells inhibits proliferation both in cultures and in growing tumors. Our results indicate that targeting the fate specification process of nascent daughter cells presents a novel avenue for developing anti-proliferation treatment for malignant brain tumors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

