IL-17 mediates protective immunity against nasal infection with Bordetella pertussis by mobilizing neutrophils, especially Siglec-F+ neutrophils
- PMID: 33976385
- PMCID: PMC8379078
- DOI: 10.1038/s41385-021-00407-5
IL-17 mediates protective immunity against nasal infection with Bordetella pertussis by mobilizing neutrophils, especially Siglec-F+ neutrophils
Erratum in
-
Correction: IL-17 mediates protective immunity against nasal infection with Bordetella pertussis by mobilizing neutrophils, especially Siglec-F+ neutrophils.Mucosal Immunol. 2021 Sep;14(5):1218-1219. doi: 10.1038/s41385-021-00417-3. Mucosal Immunol. 2021. PMID: 34079070 Free PMC article. No abstract available.
Abstract
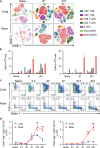
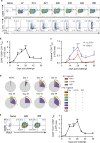
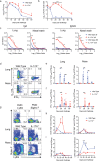
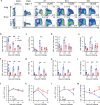
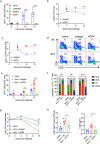
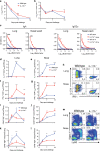
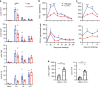
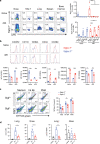
Understanding the mechanism of protective immunity in the nasal mucosae is central to the design of more effective vaccines that prevent nasal infection and transmission of Bordetella pertussis. We found significant infiltration of IL-17-secreting CD4+ tissue-resident memory T (TRM) cells and Siglec-F+ neutrophils into the nasal tissue during primary infection with B. pertussis. Il17A-/- mice had significantly higher bacterial load in the nasal mucosae, associated with significantly reduced infiltration of Siglec-F+ neutrophils. Re-infected convalescent mice rapidly cleared B. pertussis from the nasal cavity and this was associated with local expansion of IL-17-producing CD4+ TRM cells. Depletion of CD4 T cells from the nasal tissue during primary infection or after re-challenge of convalescent mice significantly delayed clearance of bacteria from the nasal mucosae. Protection was lost in Il17A-/- mice and this was associated with significantly less infiltration of Siglec-F+ neutrophils and antimicrobial peptide (AMP) production. Finally, depletion of neutrophils reduced the clearance of B. pertussis following re-challenge of convalescent mice. Our findings demonstrate that IL-17 plays a critical role in natural and acquired immunity to B. pertussis in the nasal mucosae and this effect is mediated by mobilizing neutrophils, especially Siglec-F+ neutrophils, which have high neutrophil extracellular trap (NET) activity.
© 2021. The Author(s).
Conflict of interest statement
Kingston Mills is an inventor on a patent application around a novel vaccine adjuvant and has collaborative research funding from and acts as consultant to Vaccine manufacturers. The funders had no role in the writing of the manuscript or in the decision to publish.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

