GRK3 deficiency elicits brain immune activation and psychosis
- PMID: 33976392
- PMCID: PMC8760053
- DOI: 10.1038/s41380-021-01106-0
GRK3 deficiency elicits brain immune activation and psychosis
Erratum in
-
Correction: GRK3 deficiency elicits brain immune activation and psychosis.Mol Psychiatry. 2022 Mar;27(3):1855. doi: 10.1038/s41380-021-01371-z. Mol Psychiatry. 2022. PMID: 34744164 Free PMC article. No abstract available.
Abstract
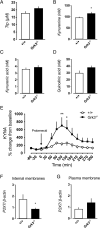
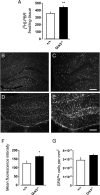
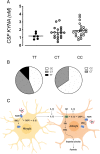
The G protein-coupled receptor kinase (GRK) family member protein GRK3 has been linked to the pathophysiology of schizophrenia and bipolar disorder. Expression, as well as protein levels, of GRK3 are reduced in post-mortem prefrontal cortex of schizophrenia subjects. Here, we investigate functional behavior and neurotransmission related to immune activation and psychosis using mice lacking functional Grk3 and utilizing a variety of methods, including behavioral, biochemical, electrophysiological, molecular, and imaging methods. Compared to wildtype controls, the Grk3-/- mice show a number of aberrations linked to psychosis, including elevated brain levels of IL-1β, increased turnover of kynurenic acid (KYNA), hyper-responsiveness to D-amphetamine, elevated spontaneous firing of midbrain dopamine neurons, and disruption in prepulse inhibition. Analyzing human genetic data, we observe a link between psychotic features in bipolar disorder, decreased GRK expression, and increased concentration of CSF KYNA. Taken together, our data suggest that Grk3-/- mice show face and construct validity relating to the psychosis phenotype with glial activation and would be suitable for translational studies of novel immunomodulatory agents in psychotic disorders.
© 2021. The Author(s).
Conflict of interest statement
MB is employed by AstraZeneca. D-SC is a scientific advisory board member to Peptron Inc. CMS is a scientific advisor to Outermost Inc. (MA, USA). All other authors declare they have no competing interests.
Figures


References
-
- Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Snapshot of activated G proteins at the membrane: the Galphaq-GRK2-Gbetagamma complex. Science. 2005;310:1686–1690. - PubMed
-
- Erdtmann-Vourliotis M, Mayer P, Ammon S, Riechert U, Hollt V. Distribution of G-protein-coupled receptor kinase (GRK) isoforms 2, 3, 5 and 6 mRNA in the rat brain. Brain Res Mol Brain Res. 2001;95:129–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

