Postoperative acute kidney injury in adult non-cardiac surgery: joint consensus report of the Acute Disease Quality Initiative and PeriOperative Quality Initiative
- PMID: 33976395
- PMCID: PMC8367817
- DOI: 10.1038/s41581-021-00418-2
Postoperative acute kidney injury in adult non-cardiac surgery: joint consensus report of the Acute Disease Quality Initiative and PeriOperative Quality Initiative
Abstract
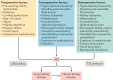
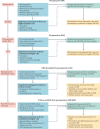
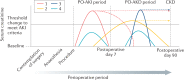
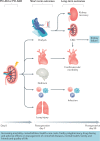
Postoperative acute kidney injury (PO-AKI) is a common complication of major surgery that is strongly associated with short-term surgical complications and long-term adverse outcomes, including increased risk of chronic kidney disease, cardiovascular events and death. Risk factors for PO-AKI include older age and comorbid diseases such as chronic kidney disease and diabetes mellitus. PO-AKI is best defined as AKI occurring within 7 days of an operative intervention using the Kidney Disease Improving Global Outcomes (KDIGO) definition of AKI; however, additional prognostic information may be gained from detailed clinical assessment and other diagnostic investigations in the form of a focused kidney health assessment (KHA). Prevention of PO-AKI is largely based on identification of high baseline risk, monitoring and reduction of nephrotoxic insults, whereas treatment involves the application of a bundle of interventions to avoid secondary kidney injury and mitigate the severity of AKI. As PO-AKI is strongly associated with long-term adverse outcomes, some form of follow-up KHA is essential; however, the form and location of this will be dictated by the nature and severity of the AKI. In this Consensus Statement, we provide graded recommendations for AKI after non-cardiac surgery and highlight priorities for future research.
© 2021. The Author(s).
Conflict of interest statement
The Acute Disease Quality Initiative (ADQI)-24 and the PeriOperative Quality Initiative (POQI)-7 Conference was supported by unrestricted education grants from the following companies: Baxter Inc, B. Braun Melsungen, BioMérieux SA AG, Cytosorbents Inc, Edwards Lifesciences Inc, La Jolla Pharmaceutical Inc, MediBeacon Inc, Medtronic Inc and Trevena Inc. A.Z. has received consulting and/or lecture fees from Astute Medical/BioMerieux, Fresenius and Baxter. A.Z. has received grant support from Astute Medical/BioMerieux, Fresenius and Baxter. R.M.P. has held research grants and has given lectures and/or performed consultancy work for Intersurgical, GlaxoSmithKline and Edwards Lifesciences, and holds editorial roles with the British Journal of Anaesthesia, the British Journal of Surgery and BMJ Quality and Safety. M.B. reports research funding from Baxter Inc. M.G.M. is a consultant for Edwards Lifesciences and co-inventor of a clinical hydration device (CliniQuench Ltd). A.B. was supported by NIH Research Project Grant Program R01 GM110240. T.E.M. reports research funding and is a consultant for Edwards Lifesciences. S.M.B. reports receiving fees for scientific advisory and speaking for Baxter, for scientific advisory for CNA Diagnostics, for study clinical adjudication for BioPorto, and for travel from Spectral Medical. S.M.B. is supported by a Canada Research Chair in Critical Care Nephrology. T.J.G. reports honoraria from Acacia, Edwards, Medtronic and Merck. J.L.K. reports research funds from Astute Medical, Nxstage Medical, NIH, Satellite Healthcare and consulting Fees from Astute Medical, Baxter, Sphingotec. P.T.M. has advisory board memberships with FAST Biomedical, AM-Pharma, Sphingotec. M.J. reports honoraria and research support from Baxter Healthcare Corp, AM-Pharma, CLS Behring, Fresenius and Astute Medical. M.S.C. reports honoraria from B Braun and Edwards Lifesciences and sits on the Advisory Board for Edwards Lifesciences. J.A.K. has received grant/research support from Astellas, Astute Medical, Baxter, bioMérieux, Cytosorbents, RenalSense, consulting fees from Astellas, Astute Medical, Baxter, bioMérieux, Cytosorbents, RenalSense, DaVita, Fresenius, Jafron, Mallinckrodt, NxStage, Potrero, and has licensing of intellectual property for Astute Medical and Cytosorbents. M.O. declared having received consultancy fees from NxStage, speaker honoraria from Fresenius Medical Care and research support from LaJolla Pharma. K.D.L. declared having received consultancy fees from bioMérieux, speaker honoraria from Baxter, and stock options from Amgen. J.R.P. declared having received consultancy fees from MediBeacon, Nikkiso Europe GmbH, and Quark Pharmaceuticals; speaker honoraria from Baxter, Fresenius Medical Care, and Nikkiso Europe GmbH; and research support from bioMérieux. A.D.S. acts as a consultant for Edwards Lifesciences, FAST Biomedical and Astellas Pharma. L.G.F. has received honoraria and research support from Astute Medical, La Jolla Pharmaceuticals, Medibeacon, Baxter and Fresenius. The other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

